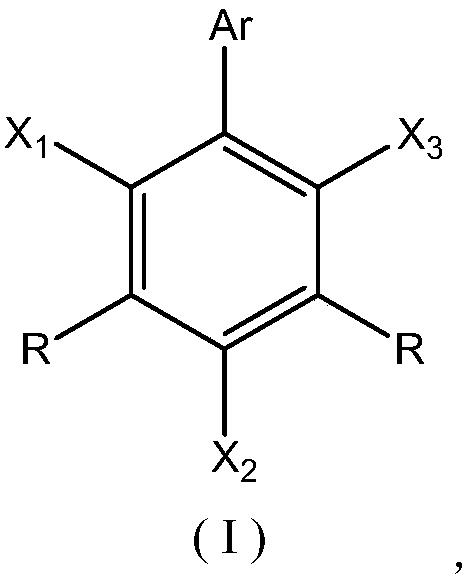
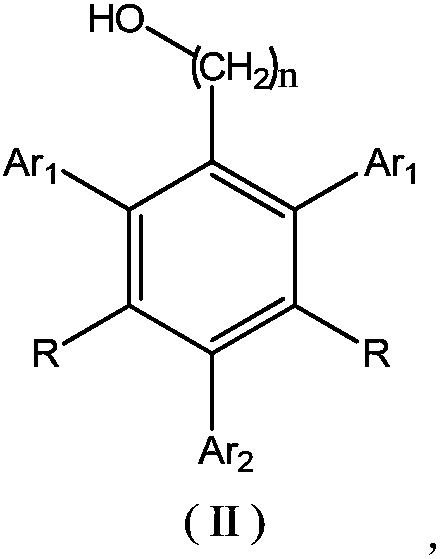
Flame-retardant polyether polyol as well as preparation method and application thereof
A polyether polyol and flame retardant technology, which is applied in the field of flame retardant polyether polyol and its preparation, can solve problems such as the decline of flame retardant performance, and achieve the effect of meeting the requirements of high flame retardant grade and meeting the requirements of flame retardant grade.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
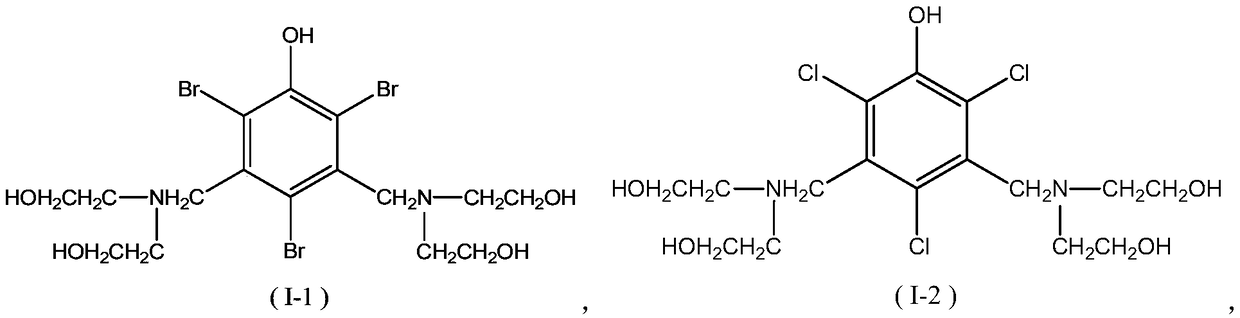
[0052] The present embodiment provides a kind of Mannich base, has the structure shown in following formula (I-1):
[0053]
[0054] Mannich base shown in formula (I-1) is prepared by following steps:
[0055] (1) Add 52.6g of diethanolamine into a 250mL round bottom flask, stir, and heat to 40-45°C; then add 15g of paraformaldehyde (molecular weight: 30) into the flask in four batches. The interval time is 15 minutes, and the temperature is controlled at 50-55°C during the paraformaldehyde feeding process;
[0056] After adding paraformaldehyde, continue to react at 50°C for 3 hours to generate 3-hydroxyethyl-1,3-oxazolidine;
[0057] Raise the temperature to 100°C, dehydrate the generated 3-hydroxyethyl-1,3-oxazolidine under reduced pressure, so that the 3-hydroxyethyl-1,3-oxazolidine has a water content of ≤0.5%;
[0058] (2) Cool down to 60-65°C, add 83g of 2,4,6-tribromophenol (phenyl group represented by formula (I'-1) to 3-hydroxyethyl-1,3-oxazolidine compound), 2...
Embodiment 2
[0062] The present embodiment provides a kind of Mannich base, has the structure shown in following formula (I-2):
[0063]
[0064] Mannich base shown in formula (I-2) is prepared by following steps:
[0065] (1) Add 56.5g of diethanolamine into a 250mL round bottom flask, stir, and heat to 40-45°C; then add 15g of paraformaldehyde (molecular weight: 30) into the flask in four batches. The interval time is 15 minutes, and the temperature is controlled at 55-60°C during the paraformaldehyde feeding process;
[0066] After adding paraformaldehyde, continue to react at 55°C for 3 hours to generate 3-hydroxyethyl-1,3-oxazolidine;
[0067] Raise the temperature to 100°C, dehydrate the generated 3-hydroxyethyl-1,3-oxazolidine under reduced pressure, so that the 3-hydroxyethyl-1,3-oxazolidine has a water content of ≤0.5%;
[0068] (2) Cool down to 60-65°C, add 49.4g of 2,4,6-trichlorophenol (the phenyl group shown in formula (I'-2) to 3-hydroxyethyl-1,3-oxazolidine compound)),...
Embodiment 3
[0073] The present embodiment provides a kind of Mannich base, has the structure shown in following formula (I-3):
[0074]
[0075] Mannich base shown in formula (I-3) is prepared by following steps:
[0076] (1) Add 52.6g of diethanolamine into a 250mL round bottom flask, stir, and heat to 40-45°C; then add 15.75g of paraformaldehyde (molecular weight: 30) into the flask in four batches, each batch The feeding interval is 15 minutes, and the temperature is controlled at 50-55°C during the paraformaldehyde feeding process;
[0077] After adding paraformaldehyde, continue to react at 55°C for 3 hours to generate 3-hydroxyethyl-1,3-oxazolidine;
[0078] Raise the temperature to 100°C, dehydrate the generated 3-hydroxyethyl-1,3-oxazolidine under reduced pressure, so that the 3-hydroxyethyl-1,3-oxazolidine has a water content of ≤0.5%;
[0079] (2) Cool down to 60-65°C, add 71.6g of 2,6-dibromo-4-chlorophenol (formula (I'-3 shown in phenyl compound)), 2,6-dibromo-4-chloroph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| limiting oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com