Medicine for photodynamic therapy and chemotherapy of cancer and preparation method thereof
A technology of photodynamic therapy and photodynamic therapy, applied in the field of medicine, to achieve the effect of single molecular weight distribution, easy control and use, and avoid π-π stacking and aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Synthesis of PDP
[0051]
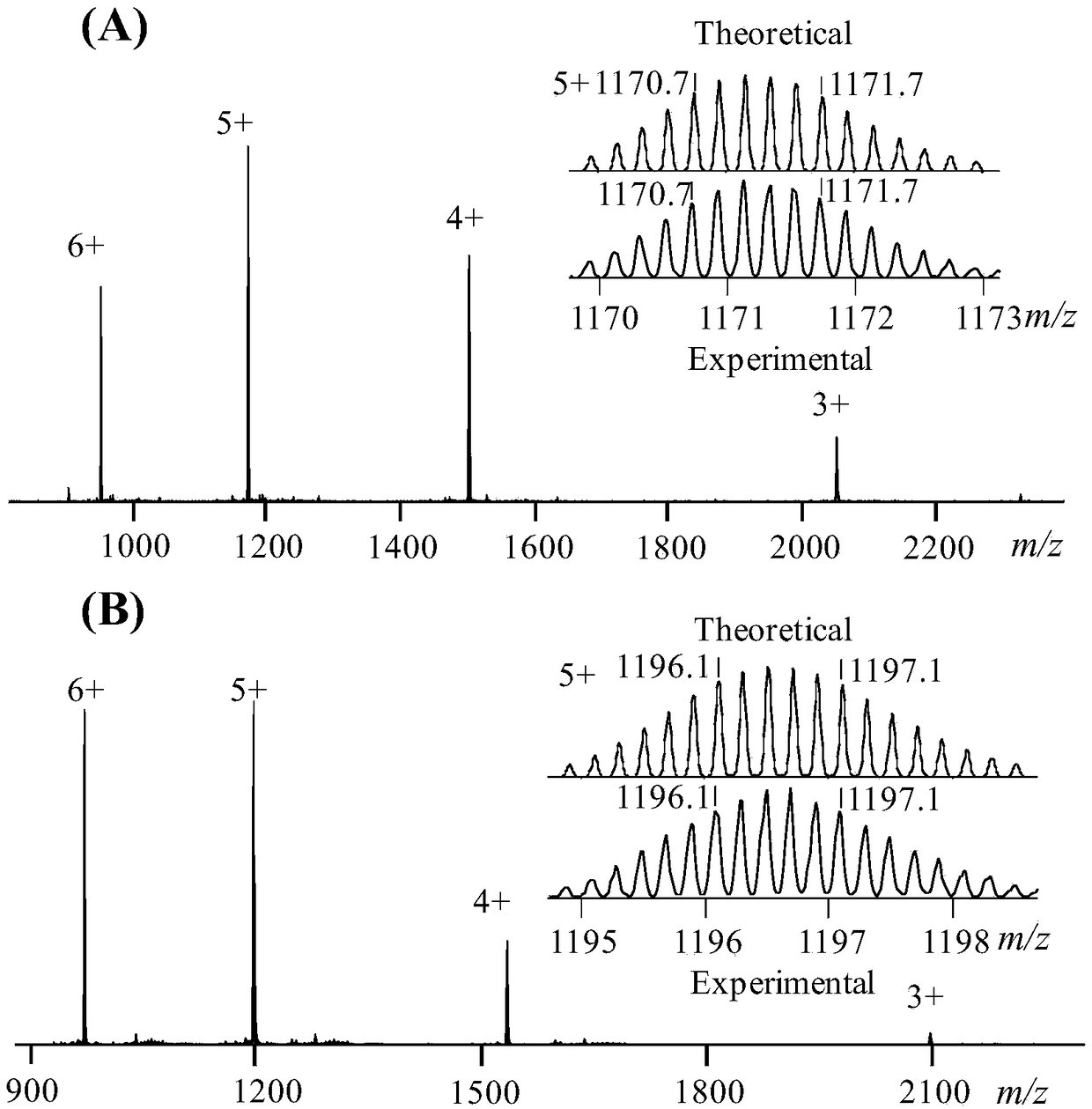
[0052] Weigh 16.2 μmol of 5,10,15,20-tetrakis(3-pyridyl)porphyrin (P) and 4,4'-bis(trans-bis(triethylphosphine)(trifluoromethane)platinum) Benzophenone (DP) 32.4 μmol, the above substances were dissolved in dimethyl sulfoxide, reacted at 80 ° C for 12 h, after cooling to room temperature, an excess of diethyl ether was added to form a precipitate, filtered, washed with ether, It was then dried in vacuo to give a dark brown solid (51.5 mg, 96.4%).
[0053] 1 H NMR (δppm): δ9.60(m,8H,Py-H a ),δ9.33(d,8H,Py-H b ),δ8.94 (m,24H,Py-H d ,P-H e ),δ8.28(t,8H,Py-H c ),δ7.63(d,16H,Ph-H 2 ),δ 7.20-7.30(m,16H,Ph-H 1 ),δ-3.13(s,4H,P-H f ).
[0054] 31 P{1H}NMR: δ=12.64ppm
[0055] ESI-MS ( figure 1 (A)): 6600Da
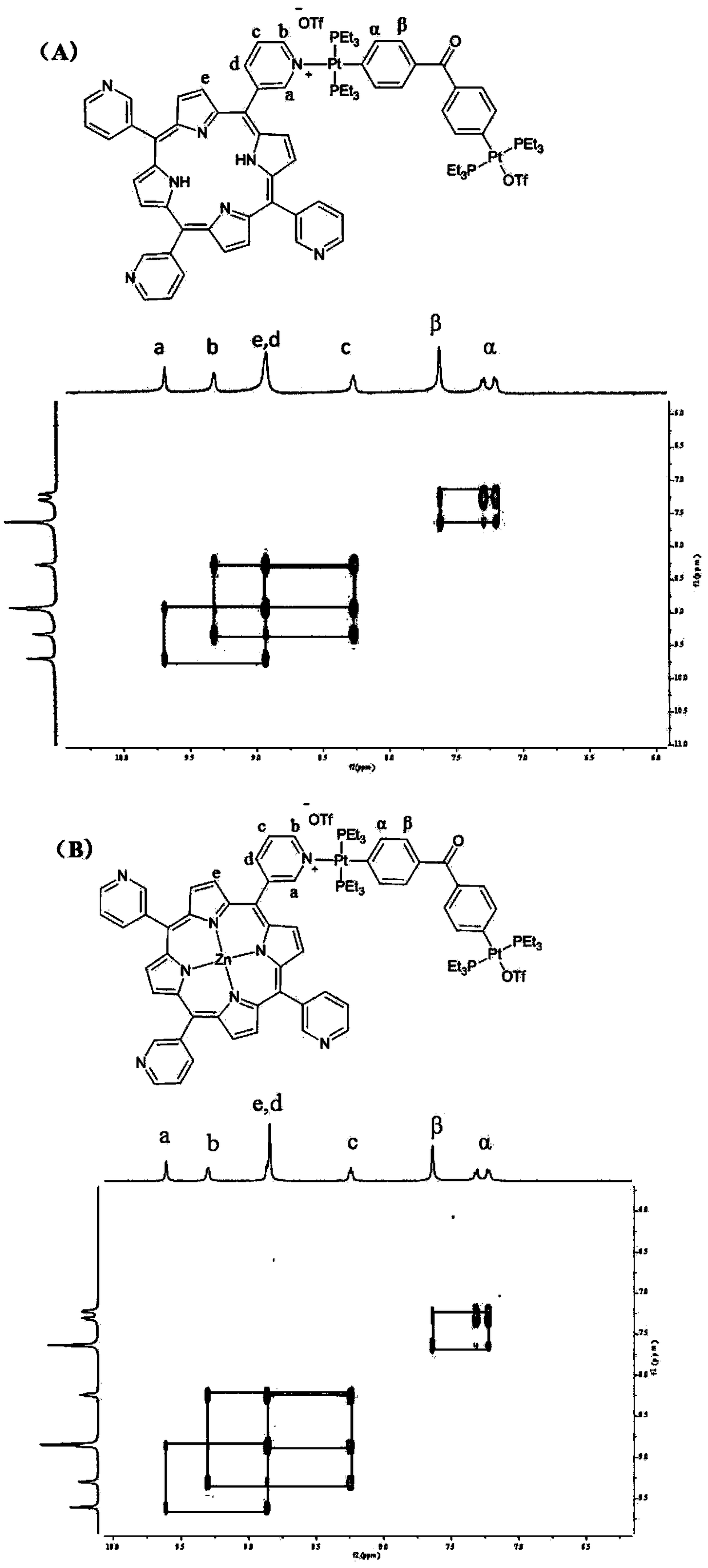
[0056] 2D correlation spectrum (2D COZY) ( figure 2 (A))
Embodiment 2
[0058] Synthesis of ZPDP
[0059]
[0060] Weigh 14.7 μmol of zinc 5,10,15,20-tetrakis(4-pyridyl)-21H, 23H-porphyrin (ZP) and 4,4'-bis(trans-bis(triethylphosphine) (tri Fluoromethane) platinum) benzophenone (DP) 29.4 μmol, the above substances were dissolved in dimethyl sulfoxide, reacted at 80 ° C for 12 h, after cooling to room temperature, an excess of diethyl ether was added to form a precipitate, Filtration, washing with ether, and drying in vacuo gave a purple-black solid (47.0 mg, 95.1%).
[0061] 1 H NMR: δ9.60(s,8H,Py-H a ),δ9.29-9.30(d,8H,Py-H b ),δ8.84-8.87 (m,24H,Py-H d ,ZP-H e ),δ8.22-8.25(t,8H,Py-H c ),δ7.64(d,16H,Ph-H 2 ),δ 7.21-7.32(m,16H,Ph-H 1 )
[0062] 31 P{1H}NMR: 12.73ppm
[0063] ESI-MS ( figure 1 (B)): 6732Da
[0064] 2D correlation spectrum (2D COZY) ( figure 2 (B))
Embodiment 3
[0066] Preparation of supramolecular cage-loaded nanoparticles
[0067] Preparation of Supramolecular Cage-loaded Nanoparticles PDP NPs and ZPDP NPs
[0068] Add 5 mL of acetone solution containing 6.0 mg of supramolecular cage (PNP or ZPNP), mPEG-b-PEBP (25.0 mg) and RGD-PEG-b-PEBP (5.0 mg) dropwise into 20 mL of Milli-Q water and stir vigorously , vacuum dried. After 5 minutes of sonication, a well-dispersed nanoparticle suspension was obtained.
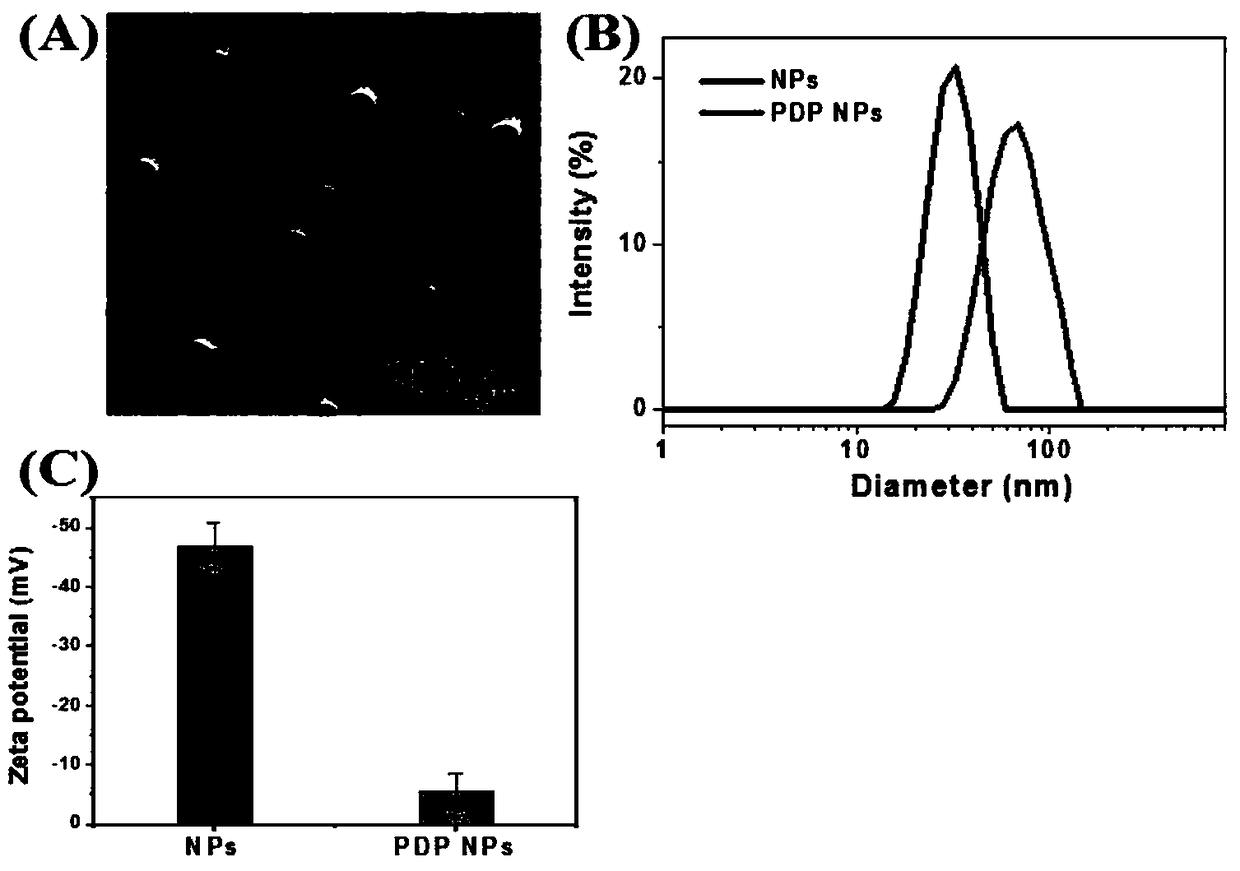
[0069] The morphology and size of the nanoparticles were studied by transmission electron microscopy (TEM) and dynamic laser scattering (DLS), see image 3 ,As can be seen, image 3 As shown in A, spherical PDP NPs with diameters ranging from 30 to 90 nm were observed in the dry state. Due to the hydration of NP, from DLS( image 3 B) A slightly larger hydrophilic diameter is noted. An increase in diameter from 35.7 nm to 61.8 nm was observed after loading the PDP cage into NPs, indicating that the amphiphilic polymer success...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com