Water-soluble chitosan antibacterial derivative and preparation method thereof
A water-soluble chitosan, chitosan technology, applied in the directions of botanical equipment and methods, chemicals for biological control, biocides, etc. Functionalization and other issues to achieve the effect of ensuring antibacterial and antibacterial properties, ensuring biosafety, and enhancing water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
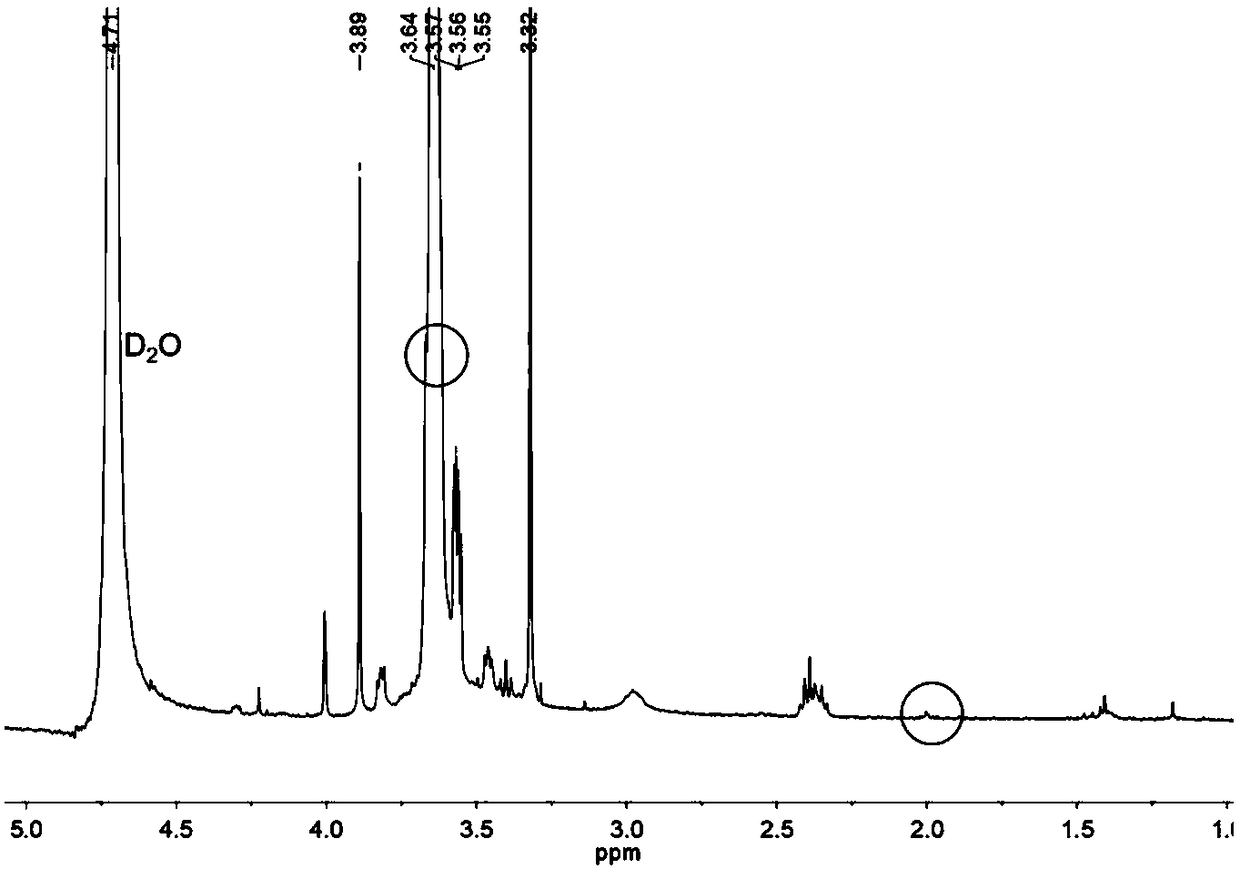
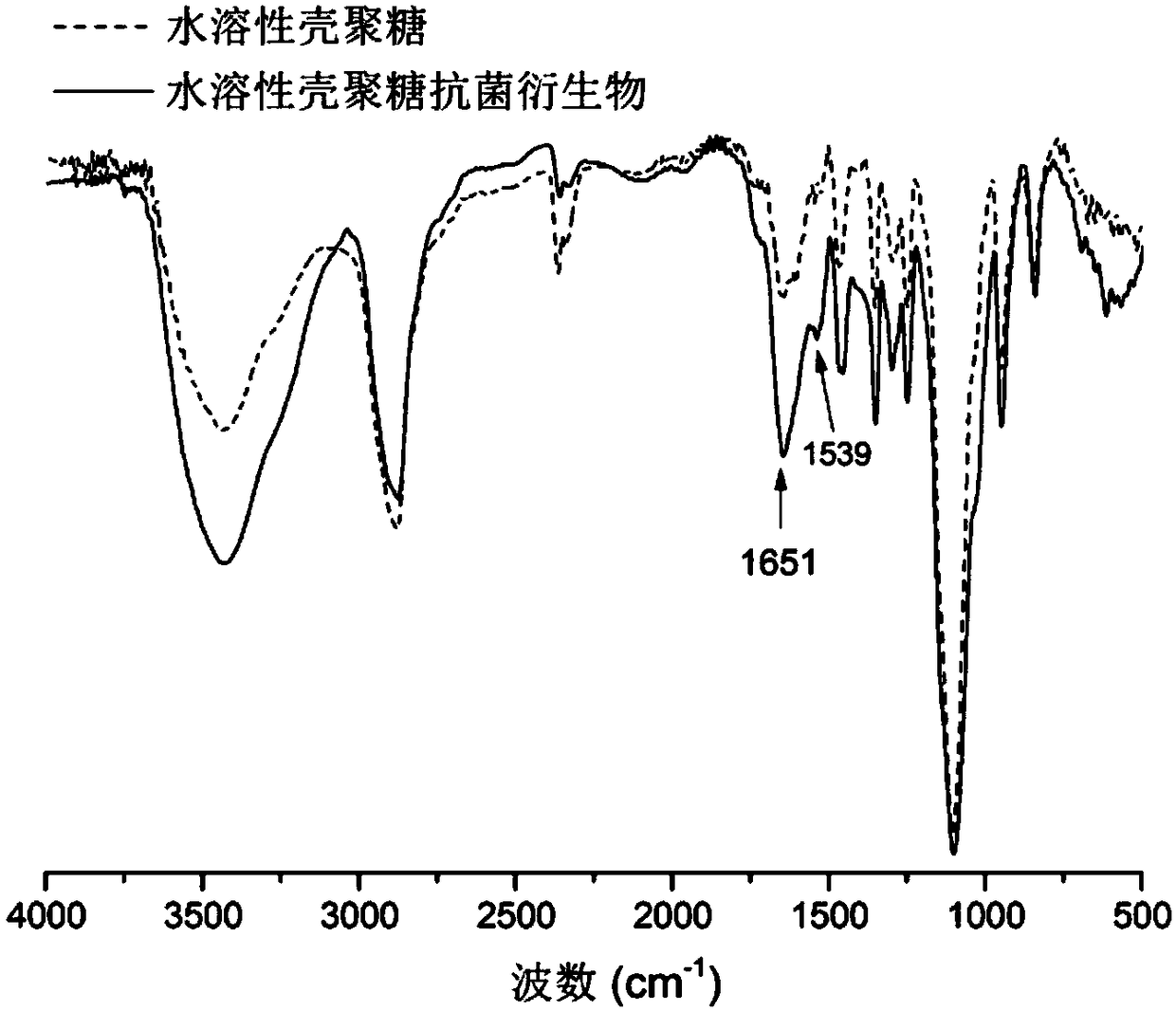
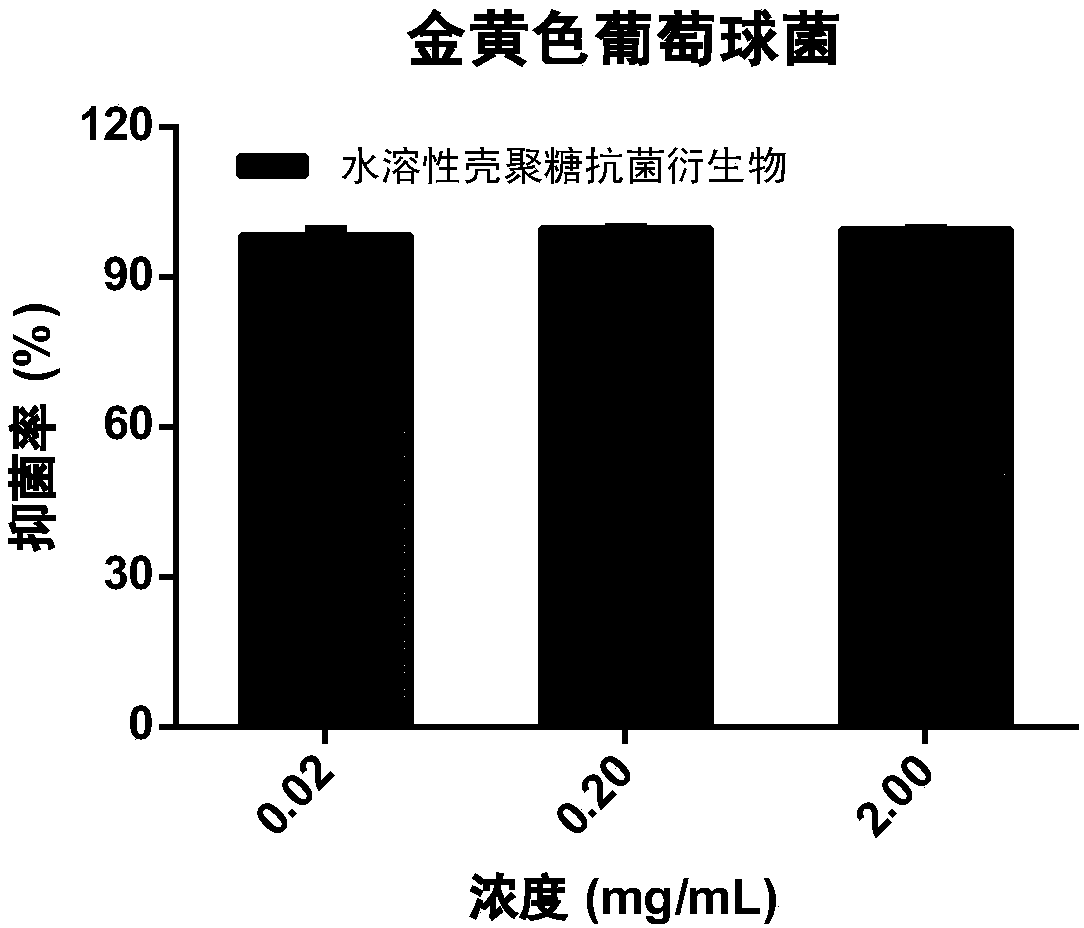
Image
Examples
Embodiment 1
[0040] (1) Preparation of polyethylene glycol modified chitosan
[0041] Weigh 0.16 grams of molecular weight 2×10 5 Chitosan with a Da deacetylation degree of 99% was added to 10mL MES buffer (25mM, pH=4.90), and 0.1mL HCl was dropped into 0.1mL HCl and stirred at room temperature for half an hour to completely dissolve the chitosan, thereby obtaining a mass volume percentage concentration of 1.6% homogeneous solution; then 20 mL of a mixed solution of carboxypolyethylene glycol monomethyl ether, NHS and EDC HCl activated at room temperature for 1 hour (the solvent is MES buffer solution of 25 mM pH=4.90) was added to the above reaction solution , keep stirring and reacting at room temperature for 24 hours, wherein the ratio of the amount of chitosan, carboxypolyethylene glycol monomethyl ether, NHS, EDC·HCl is 1:1:2:2; Polyethylene glycol monomethyl ether and other substances such as hydroxylamine hydrochloride terminate the reaction, and then the reaction solution is trans...
Embodiment 2
[0045] (1) Preparation of polyethylene glycol modified chitosan
[0046] Weigh 0.10 grams of molecular weight 10 6 Chitosan with a Da deacetylation degree of 80% was added to 100mL MES buffer (10mM pH=7.50), and 0.4mL CH 3 COOH was stirred at room temperature for half an hour to completely dissolve chitosan, thereby obtaining a homogeneous solution with a mass volume percentage concentration of 0.1%; then carboxypolyethylene glycol monomethyl ether, NHS and Add 20mL of EDC·HCl mixed solution (10mM solvent, MES buffer solution with pH=7.50) into the above reaction solution, and continue to stir and react at 0°C for 72 hours, wherein chitosan, carboxypolyethylene glycol monomethyl ether, The ratio of the amount of substances of NHS and EDC·HCl is 20:2:1:1; after the reaction is completed, add hydroxylamine hydrochloride with the amount of carboxypolyethylene glycol monomethyl ether and other substances to terminate the reaction, and then transfer the reaction solution to the in...
Embodiment 3
[0050] (1) Preparation of polyethylene glycol modified chitosan
[0051] Weigh 5.00 grams of chitosan with a molecular weight of 1000Da and a degree of deacetylation of 50% and add it to 10mL of MES buffer (100mMpH=4.50), and stir at room temperature for half an hour to completely dissolve the chitosan, thereby obtaining a concentration of 50% by mass volume. Homogeneous solution; then 50mL of a mixed solution of carboxypolyethylene glycol monomethyl ether, NHS and EDC HCl activated at 35°C for 0.5 hours (the solvent is MES buffer solution of 100mM pH=4.50) was added to the above reaction solution, and Continue to stir and react at 35°C for 12 hours, wherein the ratio of the amount of chitosan, carboxypolyethylene glycol monomethyl ether, NHS, EDC·HCl is 1:10:5:5; Hydroxylamine hydrochloride in the amount of ethylene glycol monomethyl ether and other substances terminates the reaction, and then the reaction solution is transferred to a dialysis bag with a molecular weight cut-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com