Preparation method of novel anti-human papilloma viral protein
An anti-human papilloma and virus technology, applied in the direction of antiviral agents, biochemical equipment and methods, viruses, etc., can solve the problems of not meeting the market demand, low expression of natural PAP protein, and lack, etc., to achieve large-scale application Prospect, simple preparation method, effect of reducing operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0025] The preparation method of novel anti-human papillomavirus protein of the present invention comprises the following steps:
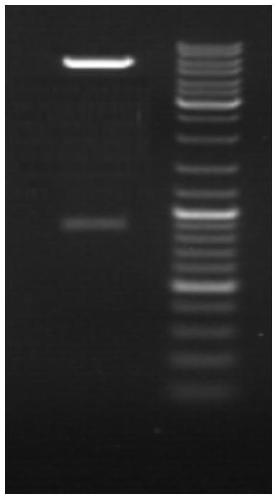
[0026] 1. According to the sequence of the PAP gene published on Genbank, the coding region of the PAP gene is 942bp in length, encoding 313 amino acids, with a signal peptide consisting of 22 amino acids at the N-terminus and a stable region consisting of 29 amino acids at the C-terminus . The amplification of the gene is used for the construction of the later eukaryotic expression vector, so after removing the 22 amino acids encoded by the 66 bases at the N-terminus, the total length is 876bp. The N-terminal mutated PAP gene sequence was synthesized, primers were designed with Prime 5.0 software, and the N-terminal mutated PAP gene was used as a template for PCR amplification. After the PCR product was recovered, it was connected to the secreted expression vector pPICk9 vector to obtain the recombinant plasmid pPICk9-PAP, and The Pichia pastoris...
experiment example 2
[0032] Experimental Example 2 Determination of the concentration of recombinant protein prepared in Example 1
[0033] BCA Micropore Quantitative Detection Method:
[0034] (1) Dilute the BSA standard: Dilute the BSA standard with the diluent consistent with the protein sample to be tested as shown in the table below.
[0035]
[0036] (2) Configure BCA working fluid:
[0037] ① Calculate the total amount of BCA working fluid:
[0038] Total amount of BCA working solution = (number of BSA standard samples + number of unknown samples) × number of multiple wells × volume of BCA working solution for each sample
[0039] The microwell detection method generally uses 3 duplicate wells, and the volume of BCA working solution for each sample is 200 μL
[0040] ②According to the calculated total amount of BCA working solution required, prepare BCA working solution with BCA-A and BCA-B according to the volume ratio of 50:1, and mix well.
[0041] (3) Quantitative detection:
[...
experiment example 3
[0048] Experimental Example 3 Western blot analysis of natural PAP protein and recombinant PAP protein
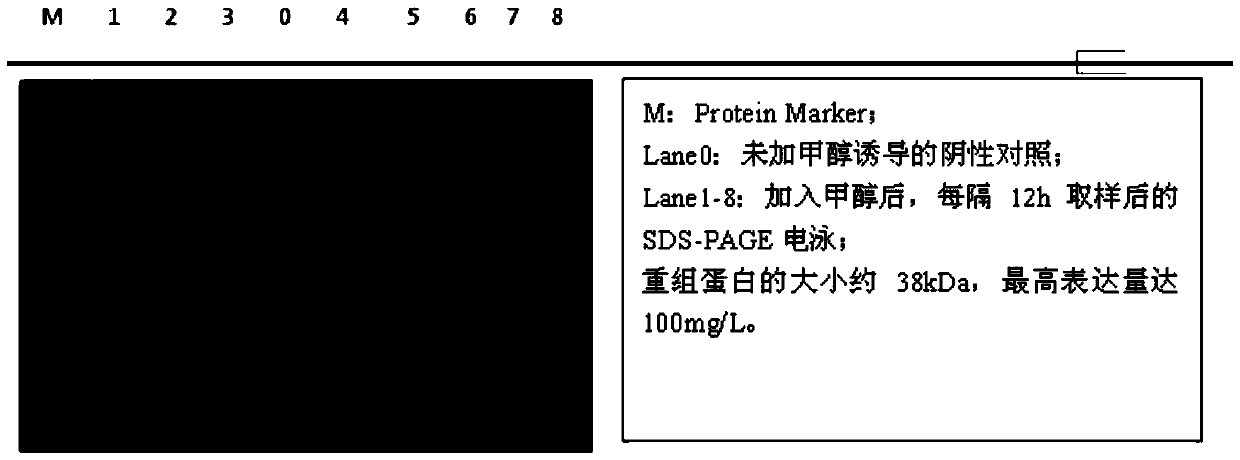
[0049] The natural and the recombinant PAP protein prepared in Example 1 were subjected to SDS-PAGE electrophoresis. After the electrophoresis, the gel was placed on the transfer buffer for rinsing, and the protein was transferred from the gel to the nitrocellulose membrane (NC); Stain the NC membrane with red, and then use PBST to wash off the color; transfer the membrane to the blocking solution to block; place the NC membrane in the previous step in the primary antibody diluted with PBST and incubate at 37°C for 1 hour, and then wash with PBST three times; Put the NC membrane from the previous step into the secondary antibody diluted with PBST, incubate at room temperature for 1 h, and then wash three times with PBST; under dark conditions, place the NC membrane in alkaline phosphatase containing NBT and BCIP for color development, and then Rinse with water to terminate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com