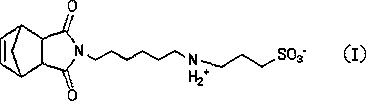
Norbornene zwitterionic monomer and preparation method thereof
A technology of norbornene and zwitterions, which is applied in the field of norbornene compounds, can solve the problems of unstable chemical degradation of matrix membranes, and achieve the effects of less environmental pollution, high reactivity, and mild preparation reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
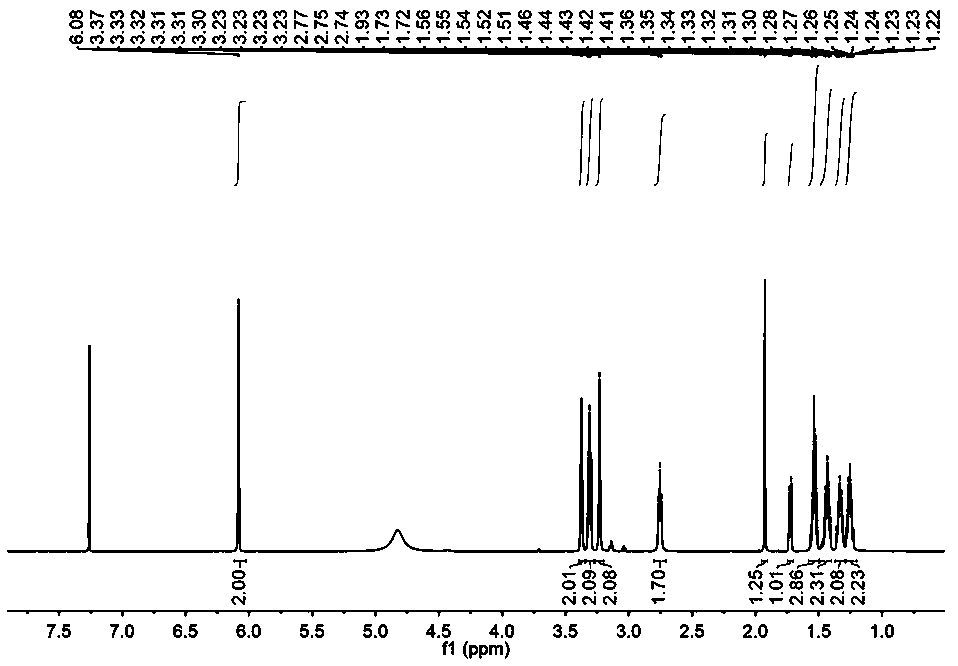
[0038] Dissolve norbornene diacid anhydride (10.0265g, 61.08mmol) in 100mL of glacial acetic acid, heat to 125°C, add 1,6-hexanediamine (21.2934g, 183.32mmol), and heat to reflux for 3h to obtain a transparent solution .
[0039] The transparent solution obtained above was distilled under reduced pressure to remove glacial acetic acid. After cooling to room temperature, 100 mL of deionized water was added, extracted three times with 100 mL of dichloromethane, and the organic layer was collected and washed three times with 50 mL of deionized water.
[0040] Collect the remaining aqueous layer after extraction and separation, adjust its pH value to 9 with 1 mol / L sodium hydroxide solution, then extract with 1 L dichloromethane three times, and collect the organic layer.
[0041] The organic layers collected twice were combined, anhydrous sodium sulfate was added, dried overnight, and a clear transparent liquid was obtained by filtration. The crude product was obtained after re...
Embodiment 2
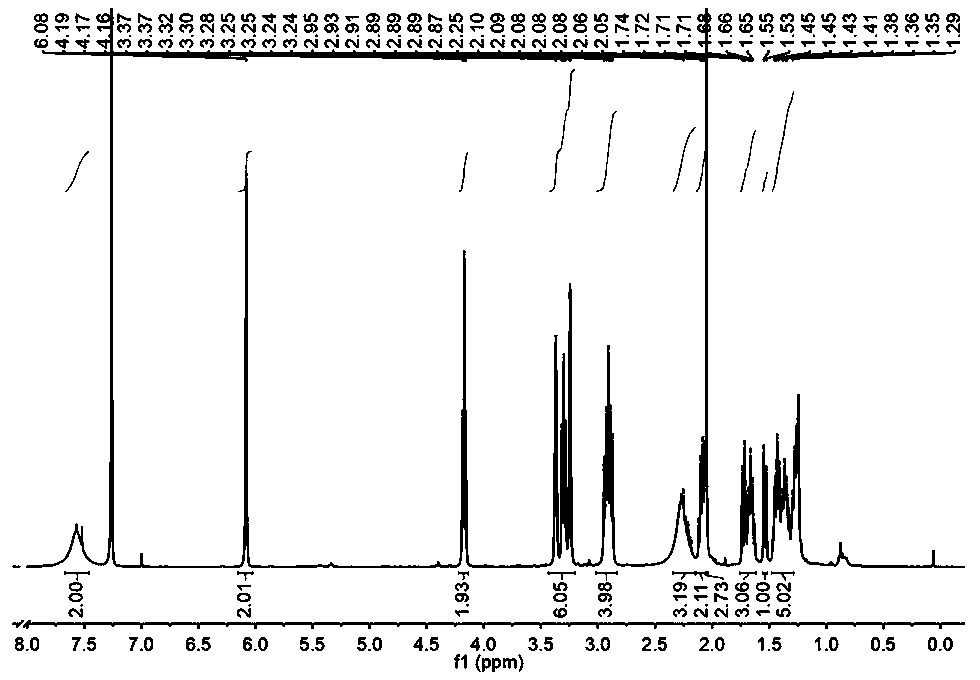
[0048] Dissolve norbornene diacid anhydride (10.0265g, 61.08mmol) in 80mL of glacial acetic acid, heat to 130°C, add 1,6-hexanediamine (14.1888g, 122.16mmol), and heat to reflux for 3h to obtain a transparent solution .
[0049] The transparent solution obtained above was distilled under reduced pressure to remove glacial acetic acid. After cooling to room temperature, 80 mL of deionized water was added, extracted three times with 80 mL of dichloromethane, and the organic layer was collected and washed three times with 40 mL of deionized water.
[0050] Collect the remaining aqueous layer after extraction and separation, adjust its pH value to 9 with 0.5 mol / L sodium hydroxide solution, then extract with 800 mL of dichloromethane three times, and collect the organic layer.
[0051] The organic layers collected twice were combined, anhydrous sodium sulfate was added, dried overnight, and a clear transparent liquid was obtained by filtration. The crude product was obtained aft...
Embodiment 3
[0056] Dissolve norbornene diacid anhydride (10.0265g, 61.08mmol) in 100mL of glacial acetic acid, heat to 135°C, add 1,6-hexanediamine (17.7361g, 152.7mmol), and heat to reflux for 4h to obtain a transparent solution .
[0057] The transparent solution obtained above was distilled under reduced pressure to remove glacial acetic acid. After cooling to room temperature, 100 mL of deionized water was added, extracted three times with 100 mL of dichloromethane, and the organic layer was collected and washed three times with 50 mL of deionized water.
[0058] Collect the remaining aqueous layer after extraction and separation, adjust its pH value to 9 with 1 mol / L sodium hydroxide solution, then extract with 1 L dichloromethane three times, and collect the organic layer.
[0059] The organic layers collected twice were combined, anhydrous sodium sulfate was added, dried overnight, and a clear transparent liquid was obtained by filtration. The crude product was obtained after rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com