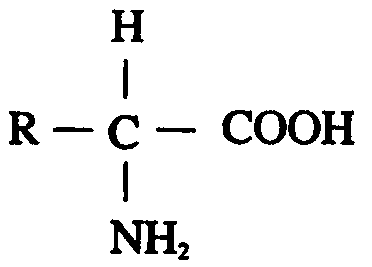Mutant of human hepatocyte growth factor (hHGF) and application of mutant
A technology of hepatocyte growth factor and mutant, applied in the field of host cells, can solve problems such as lack of serine protease activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
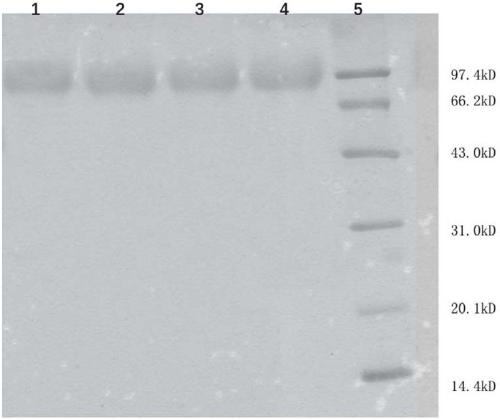
[0104] The preparation of embodiment 1.hHGF and its mutant
[0105] The amino acid sequence of native hHGF (SEQ ID NO: 1 ) can be found at NCBI Accession No. NP_000592.3. Using SEQ ID NO:1 as a template, the following three hHGF mutants were designed:
[0106] (1) Mutating Ser at position 130 of SEQ ID NO:1 to Arg to obtain hHGF mutant 130Arg-hHGF, the amino acid sequence of which is shown in SEQ ID NO:2;
[0107] (2) Mutating Ser at position 130 of SEQ ID NO:1 to His to obtain hHGF mutant 130His-hHGF, the amino acid sequence of which is shown in SEQ ID NO:3; and
[0108] (3) Mutating Ser at position 130 of SEQ ID NO:1 to Lys to obtain hHGF mutant 130Lys-hHGF, the amino acid sequence of which is shown in SEQ ID NO:4.
[0109] Using preferred codons in CHO cells, the whole gene synthesizes polynucleosides encoding native hHGF (SEQ ID NO:1) and the above three hHGF mutants (SEQ ID NO:2, SEQ ID NO:3, SEQ ID NO:4) acid, and introduce a restriction enzyme cutting site and a star...
Embodiment 2
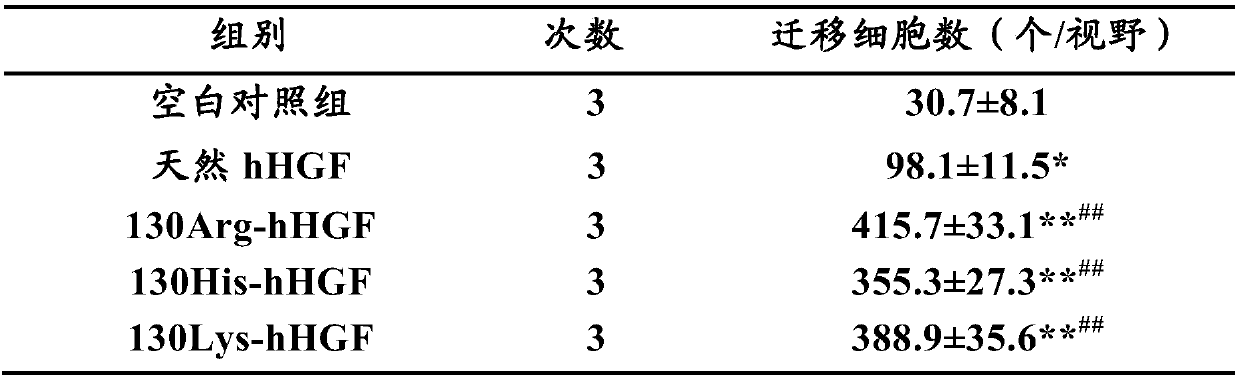
[0112] Example 2. Evaluation of in vitro biological activity of hHGF mutants and native hHGF
[0113] In this example, the effects of hHGF mutant and native hHGF on endothelial cell migration were evaluated by in vitro endothelial cell migration experiment, so as to evaluate the in vitro biological activity of hHGF mutant and native hHGF.
[0114] 1. Materials and methods
[0115] 1.1 Protein sample
[0116] The hHGF mutants prepared above (130Arg-hHGF, 130His-hHGF and 130Lys-hHGF) and natural hHGF were prepared with physiological saline to the required concentration immediately before use.
[0117] 1.2 Cell lines
[0118] ECV304 cell line (umbilical vein endothelial cells), used to test the biological activity of HGF.
[0119] 1.3 Reagents
[0120] DMEM medium: provided by Hyclone. The preparation method is as follows: take 1 bag of DMEM medium powder (the specification is 1 L), add water to dissolve and dilute to 1000 ml, and then add 2.1 g of sodium bicarbonate. Then,...
Embodiment 3
[0143] Example 3. Evaluation of the therapeutic effect of hHGF mutants and natural hHGF on rabbit lower extremity arterial ischemia model
[0144] In this example, the rabbit lower limb arterial ischemia model was used to evaluate the effect of hHGF mutant and natural hHGF on the re-formation of blood vessels and collateral circulation in the rabbit lower limb ischemia model, so as to evaluate the therapeutic effect of hHGF mutant and natural hHGF.
[0145] 1. Materials and methods
[0146] 1.1 Protein sample
[0147] The hHGF mutants prepared above (130Arg-hHGF, 130His-hHGF and 130Lys-hHGF) and natural hHGF were prepared with physiological saline to the required concentration immediately before use.
[0148] 1.2 Animal model
[0149]New Zealand male white rabbits, 12-14 months old, weighing 3.5-4.0kg, were provided by Beijing Weitong Lihua Company. According to the method described by Takeshita et al. (Therapeutics angiogenesis. A single intraarterial bolus of vascular end...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com