A method for preparing composite alkali metal niobate powder
A technology of niobate and compound alkali, which is applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, niobium compounds, etc., can solve the problems of high equipment requirements and high energy consumption, and achieve low cost, Simple operation, promoting the effect of applied research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The present embodiment provides a kind of method for preparing sodium potassium niobate powder, comprises the following steps:
[0037] (1) Add 0.2g Na 6 Nb 8 o 25 9H 2 O nanowires were dispersed in 10mL of deionized water, and 1.6g of potassium hydroxide-sodium hydroxide mixed alkali was added to make a paste, and then 0.3g of polyacrylamide was added;
[0038] Wherein, the mass ratio of potassium hydroxide and sodium hydroxide in potassium hydroxide-sodium hydroxide mixed alkali is 1:5; the average molecular weight of polyacrylamide is high molecular weight (7 million);
[0039] (2) Using the pasty mixture obtained in step (1) as a reaction precursor, heat treatment was carried out in a closed reactor (20 mL in volume) at a temperature of 200 °C for 24 hours to obtain sodium potassium niobate powder.
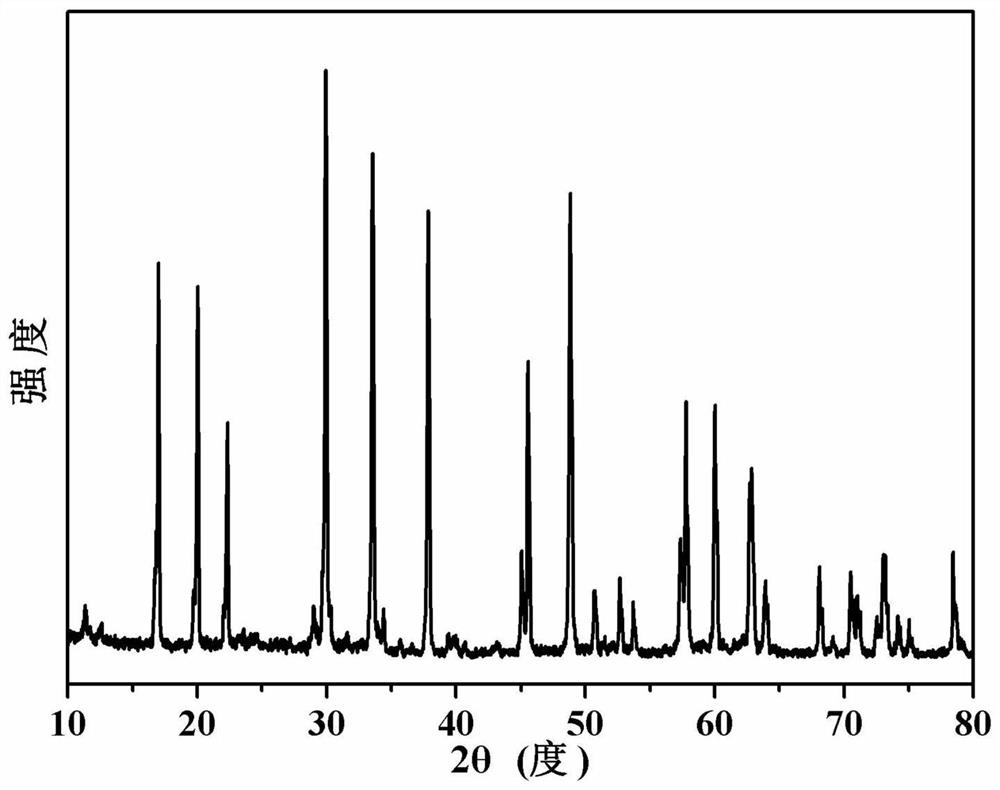
[0040] figure 1 The X-ray diffraction pattern of the sodium potassium niobate powder prepared for this example is consistent with the data reported in the literatu...
Embodiment 2
[0043] The present embodiment provides a kind of method for preparing lithium sodium niobate powder, comprises the following steps:
[0044] (1) Add 0.2g Na 6 Nb 8 o 25 9H 2 O nanowires were dispersed in 10mL of deionized water, and 1.6g of lithium hydroxide-sodium hydroxide mixed alkali was added to make a paste, and then 0.3g of polyacrylamide was added;
[0045]Wherein, the mass ratio of lithium hydroxide to sodium hydroxide in lithium hydroxide-sodium hydroxide mixed alkali is 3:1; the average molecular weight of polyacrylamide is high molecular weight (7 million);
[0046] (2) Using the pasty mixture obtained in step (1) as the reaction precursor, heat treatment was carried out in a closed reaction kettle (volume 20mL) at a temperature of 200°C for 24 hours to obtain sodium lithium niobate powder.
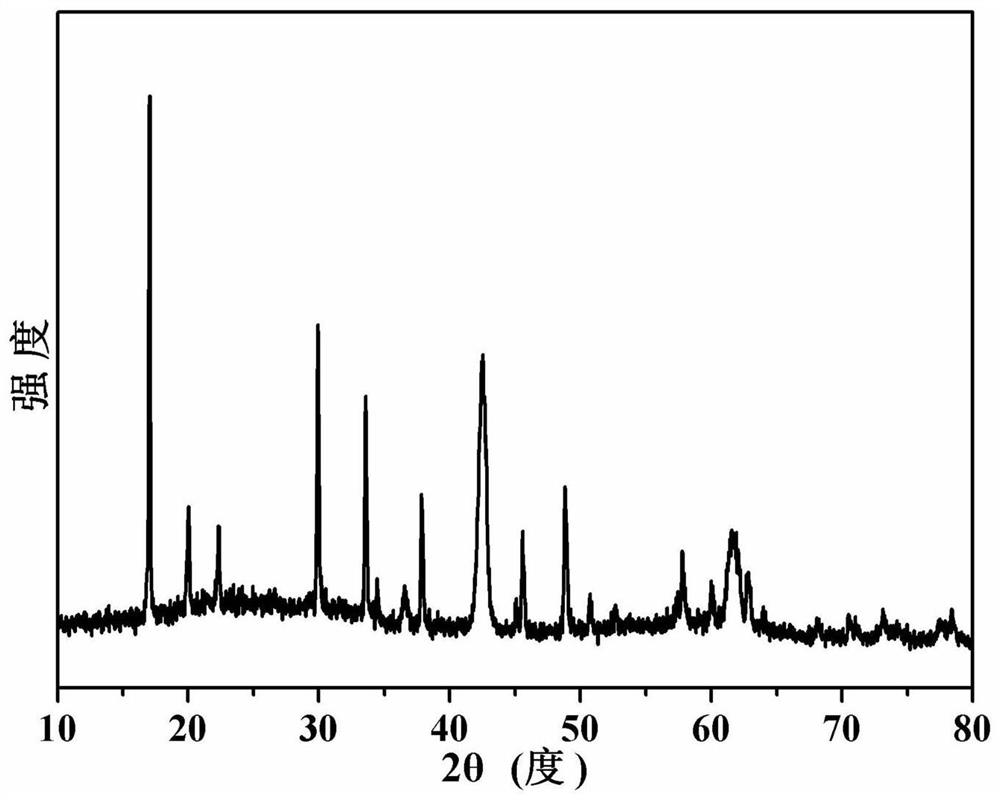
[0047] image 3 The X-ray diffraction pattern of the sodium lithium niobate powder prepared for this example is consistent with the data reported in the literature.
[0...
Embodiment 3
[0050] The present embodiment provides a kind of method for preparing sodium potassium niobate powder, comprises the following steps:
[0051] (1) Add 0.1g Na 6 Nb 8 o 25 9H 2 O nanowires were dispersed in 10mL deionized water, and 0.8g potassium hydroxide-sodium hydroxide mixed alkali was added to make a paste, and then 0.1g polyacrylamide was added;
[0052] Wherein, the mass ratio of potassium hydroxide and sodium hydroxide in the potassium hydroxide-sodium hydroxide mixed alkali is 1:1; the average molecular weight of polyacrylamide is low molecular weight (1 million);
[0053] (2) Using the pasty mixture obtained in step (1) as the reaction precursor, heat treatment was carried out in a closed reaction kettle (volume 20 mL) at a temperature of 160°C for 12 hours to obtain sodium potassium niobate powder.
[0054] Figure 5 The X-ray diffraction figure of the sodium potassium niobate powder that the present embodiment prepares; Image 6 It is the scanning electron mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com