Nasal drops for treating rhinitis and preparation method thereof
A technology for nasal drops and rhinitis, which is applied in the field of nasal drops for treating rhinitis and its preparation, can solve the problems of short drug action time, large toxic and side effects, and single pharmacological action, so as to improve nasal mucosa congestion, facilitate industrial production, The effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
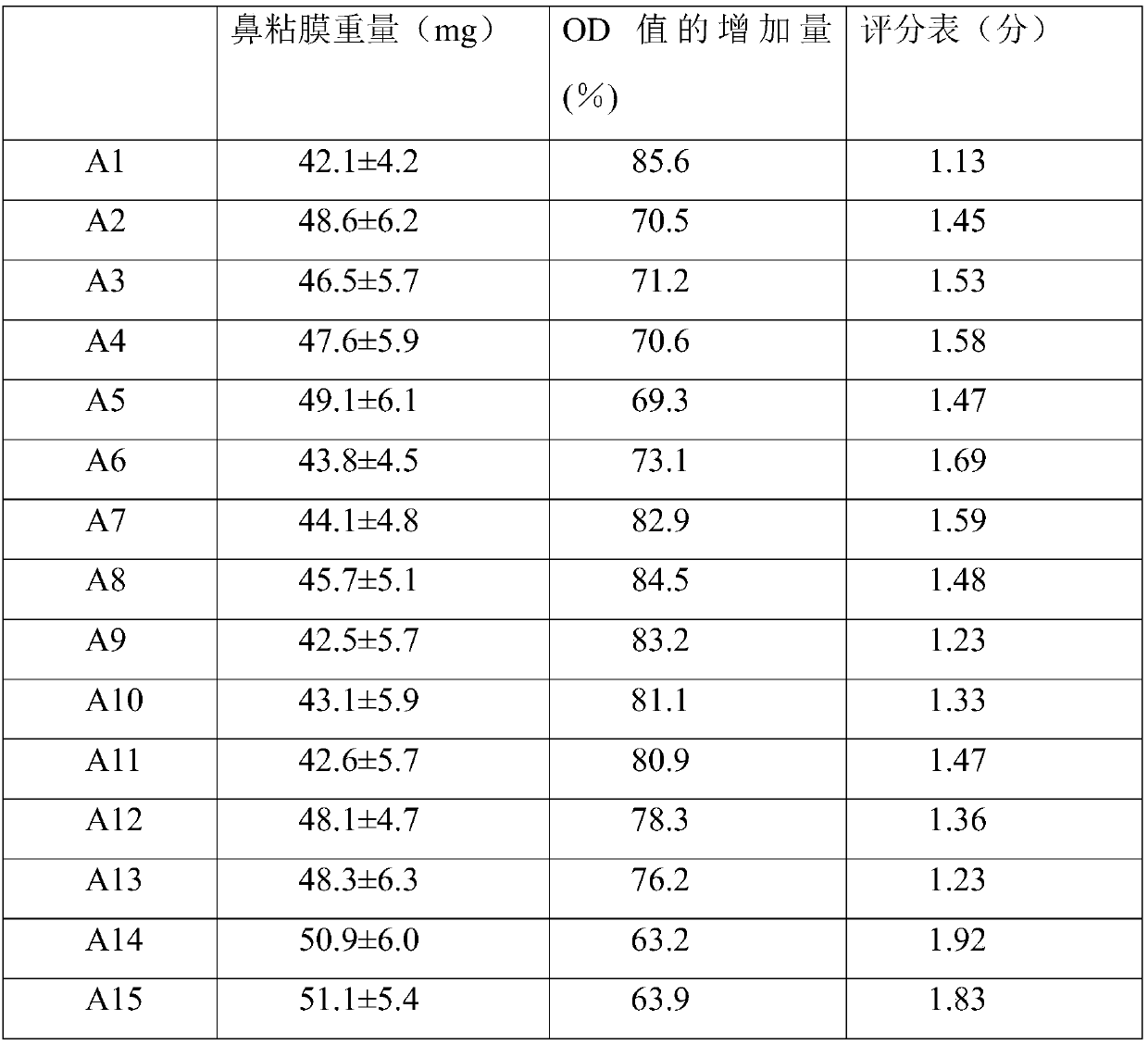
Examples
preparation example Construction
[0049] The present invention also provides a preparation method of the described nasal drops for the treatment of rhinitis, comprising the following steps:
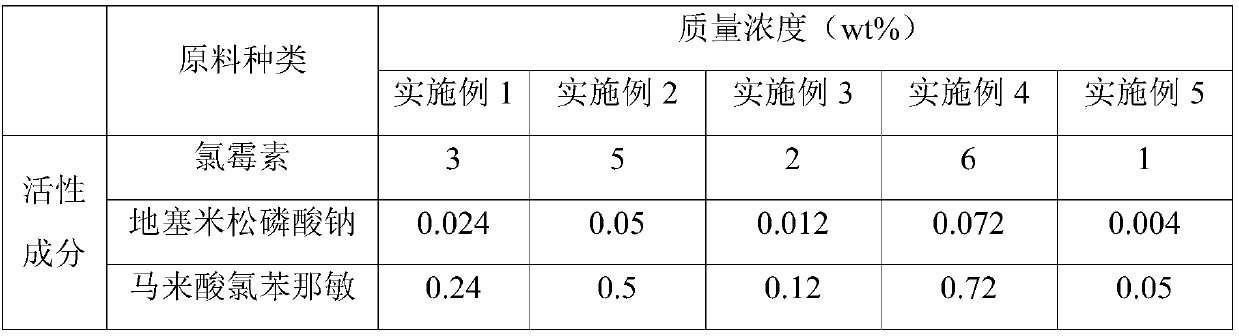
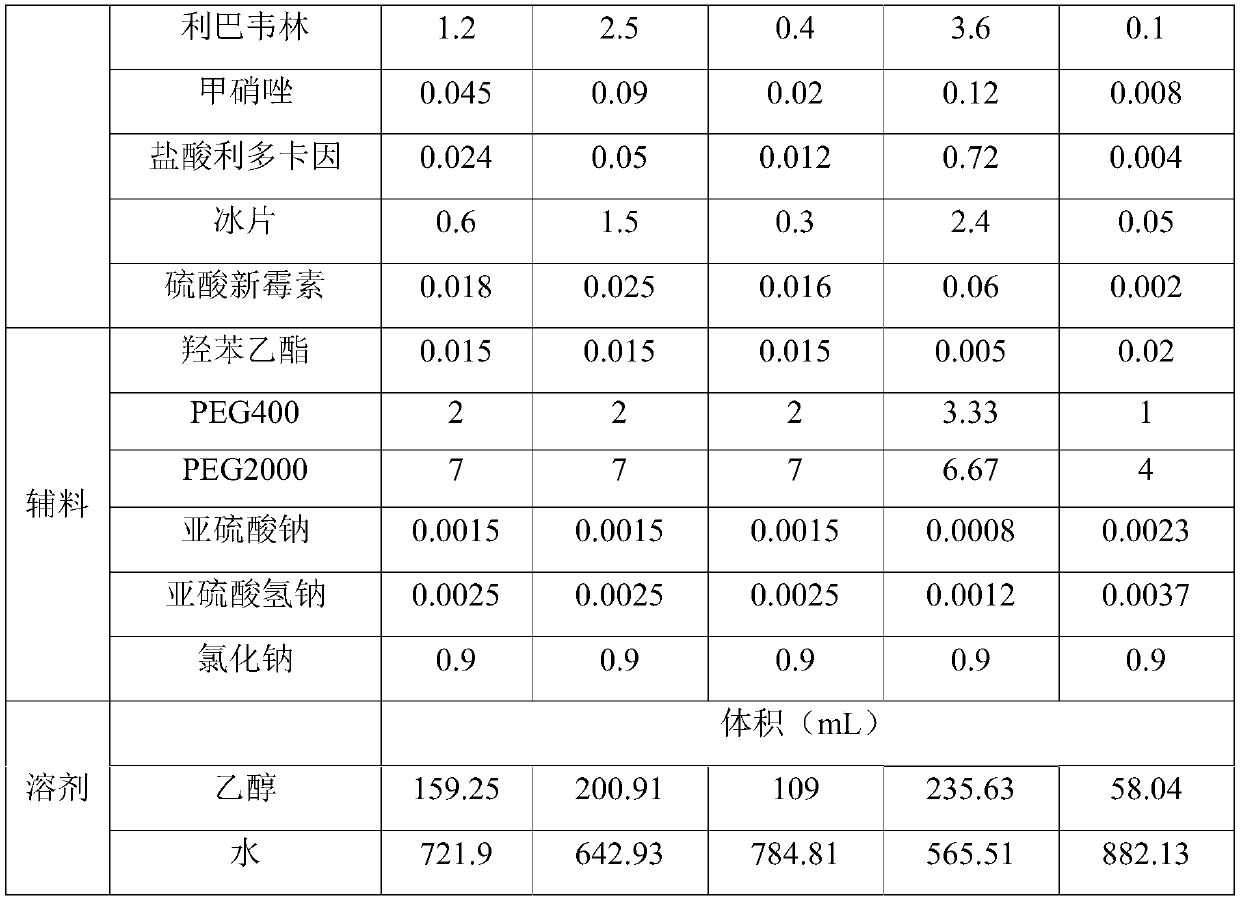
[0050] (1) Dissolve chloramphenicol, dexamethasone sodium phosphate, chlorpheniramine maleate, ribavirin, metronidazole, lidocaine hydrochloride and polyethylene glycol in a solvent of 30-50 vol%, Then pass in CO 2 to saturation, and then heated at 50 to 75 ° C for 15 to 30 min under sealing conditions to obtain medicinal solution A;
[0051] (2) Adjust the pH value of liquid A to 6.3-6.8, then add borneol, neomycin sulfate, medicinal charcoal, preservatives, stabilizers, osmotic pressure regulators and the remaining 50-70 vol% solvent, and then carry out The medicinal charcoal is removed by filtration to obtain medicinal solution B;
[0052] (3) The medicinal solution B is filtered twice with a 0.2-0.4 μm microporous membrane, and the obtained filtrate is encapsulated to obtain a nasal drop for treating rhinitis.
[0...
Embodiment 1
[0058] A kind of nasal drop for the treatment of rhinitis, its raw material composition is as shown in table 1, and preparation step is as follows:
[0059] (1) Mix 30g of chloramphenicol, 0.24g of dexamethasone sodium phosphate, 2.4g of chlorpheniramine maleate, 12g of ribavirin, 0.45g of metronidazole, 0.24g of lidocaine hydrochloride and 90g of polyethylene glycol The alcohol was dissolved in 45 vol% solvent, then passed through CO 2 to saturation, and then heated at 60°C for 25min under sealed conditions to obtain medicinal solution A;
[0060] (2) Adjust the pH value of liquid A to 6.3-6.8, then add 6g of borneol, 0.18g of neomycin sulfate, 1g of medicinal charcoal, 0.15g of ethyl paraben, 0.04g of stabilizer, 9g of sodium chloride and the rest 45vol% of solvent, then carry out circulating filtration, remove medicinal charcoal, obtain medicinal liquid B;
[0061] (3) The medicinal solution B was filtered twice with a 0.22 μm microporous membrane, and the obtained filtra...
Embodiment 2
[0064] A kind of nasal drop for the treatment of rhinitis, its raw material composition is as shown in Table 1, the preparation method of described nasal drop is the same as Example 1, and the nasal drop A2 for the treatment of rhinitis is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com