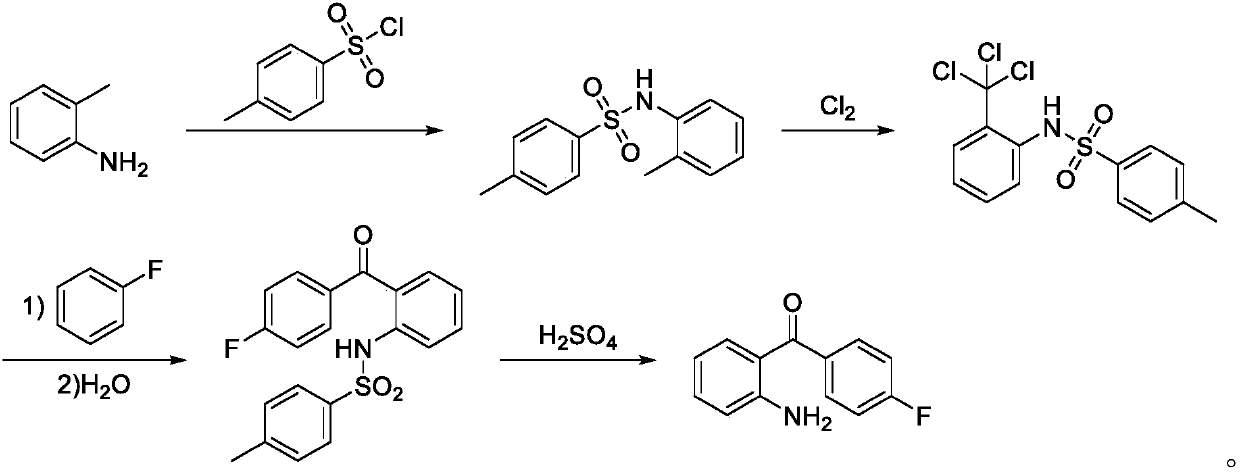
Synthetic method for 2-amino-4'-fluoro-benzophenone
A technology for benzophenone and a synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of sulfonic acid amides, etc., can solve the problems of complicated operation, unfavorable industrial production, and many reagents, etc., and achieves mild reaction conditions, Green and environmentally friendly operation, easy to operate and controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
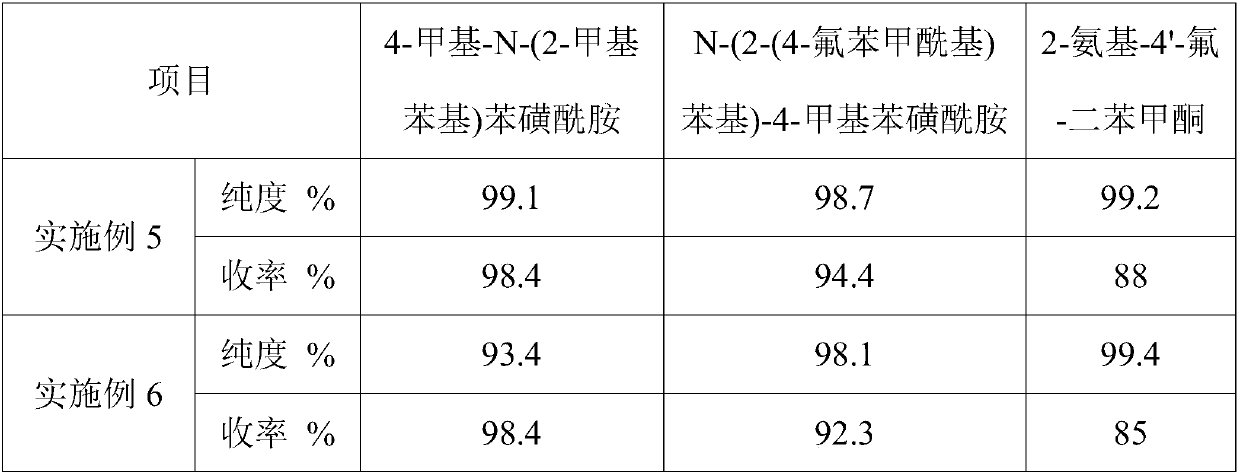
[0044] A synthetic method of 2-amino-4'-fluoro-benzophenone, comprising the steps of: performing amidation reaction of o-methylaniline and p-toluenesulfonyl chloride to obtain 4-methyl-N-(2-methylbenzene base) benzenesulfonamide; then chlorine chlorination, Friedel-Crafts reaction with fluorobenzene, and then hydrolysis to obtain N-(2-(4-fluorobenzoyl)phenyl)-4-methylbenzenesulfonamide; finally Deprotection by concentrated sulfuric acid gives 2-amino-4'-fluoro-benzophenone.
Embodiment 2
[0046] A kind of synthetic method of 2-amino-4'-fluoro-benzophenone, comprises the steps:
[0047]O-methylaniline and p-toluenesulfonyl chloride are subjected to an amidation reaction under the action of a basic catalyst to obtain 4-methyl-N-(2-methylphenyl)benzenesulfonamide;
[0048] Mix and dissolve 4-methyl-N-(2-methylphenyl)benzenesulfonamide and fluorobenzene, raise the temperature to 50°C, pass chlorine gas for 2 hours, then raise the temperature to 90°C, continue to pass chlorine gas for 6 hours, and then lower the temperature to 0 ℃, add aluminum trichloride, raise the temperature to room temperature, keep the temperature for 5 hours, then lower the temperature to 5 ℃, add water dropwise, when adding water dropwise, keep the temperature not exceeding 30 ℃, raise the temperature to 90 ℃, keep the temperature for 3h, distill to remove fluorobenzene, cool and analyze crystal, filtered, washed, dried, and recrystallized to obtain N-(2-(4-fluorobenzoyl)phenyl)-4-methylbenz...
Embodiment 3
[0051] A kind of synthetic method of 2-amino-4'-fluoro-benzophenone, comprises the steps:
[0052] Mix o-toluenesulfonyl chloride, dichloromethane, and sodium bicarbonate evenly, cool down to 5°C, add dichloromethane solution of p-toluenesulfonyl chloride dropwise, and keep the temperature not exceeding 5°C, after the dropwise addition, warm up to room temperature, keep warm for 3h, filter the filtrate, extract the organic phase with dichloromethane and water, dry, and concentrate to obtain 4-methyl-N-(2-methylphenyl)benzenesulfonate Amide, wherein the molar ratio of o-toluenesulfonyl chloride to p-toluenesulfonyl chloride is 1:3, and the molar ratio of o-toluene to sodium bicarbonate is 1:1.2;
[0053] Mix and dissolve 4-methyl-N-(2-methylphenyl)benzenesulfonamide and fluorobenzene, raise the temperature to 50°C, pass chlorine gas for 2 hours, then raise the temperature to 110°C, continue to pass chlorine gas for 4 hours, then lower the temperature to 10°C ℃, add aluminum tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com