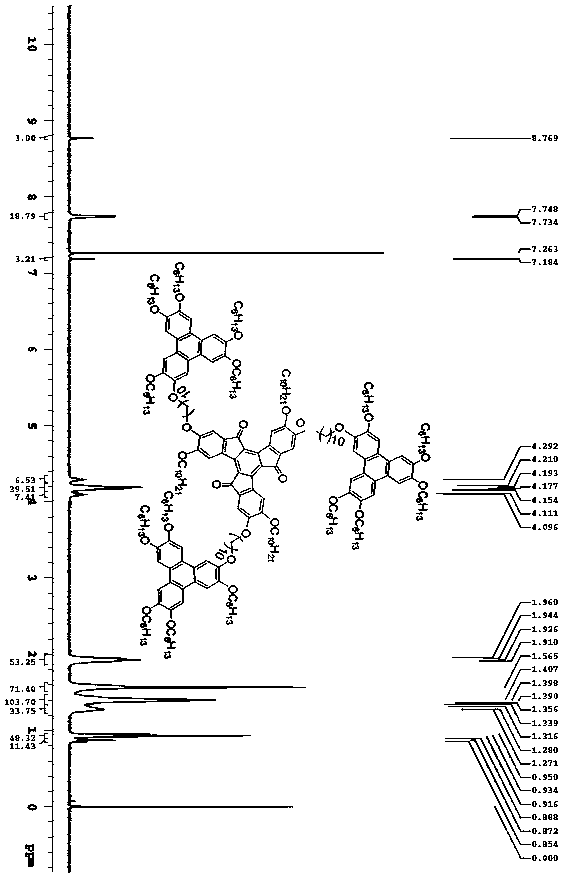
Truxeneone-benzophenanthrene disk-shaped liquid crystal compound and preparation method thereof
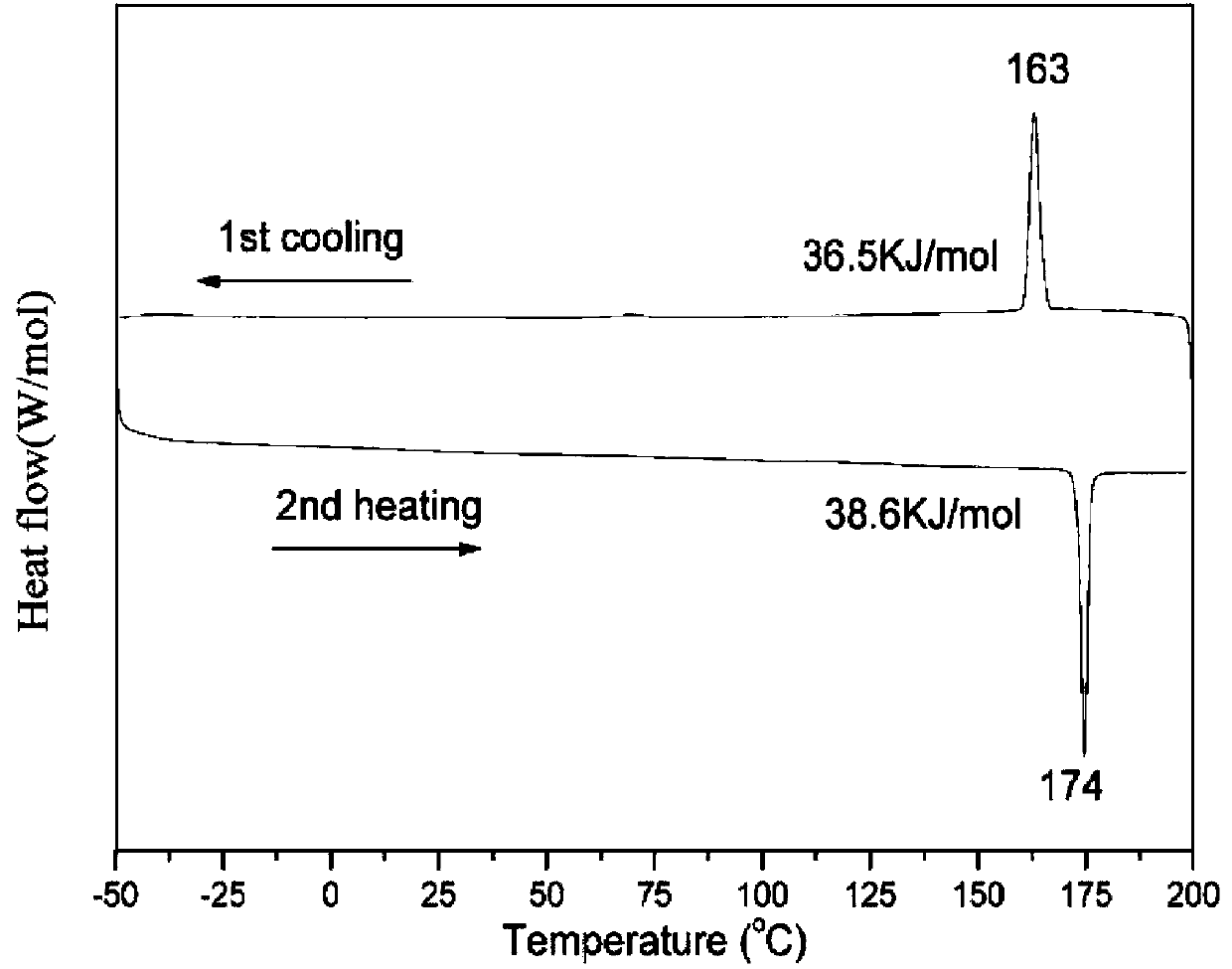
A discotic liquid crystal and tuxikenone technology, which is applied in chemical instruments and methods, liquid crystal materials, etc., can solve the problems of only 36% yield, low efficiency, etc., and achieves good chemical and thermal stability, simple operation, The effect of a wide mesogenic temperature range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of Tuoxynone-Triphenylene Discotic Liquid Crystalline Compound (TRO-6-TP)
[0040]
[0041] Monobromotriphenylene TP (OC 6 h 13 ) 5 (OCH 2 ) 6 Br (102 mg, 0.112 mmol), potassium carbonate (80 mg, 0.58 mmol), and 15 mL N,N'-dimethylformamide were added to a 25 mL round bottom flask, stirred at room temperature, and the monobromotriphenylene Completely dissolved, under the protection of an inert gas, added trihydroxytuximene compound (30 mg, 0.035 mmol), heated and stirred at 80°C, reacted for 36 h, removed the protection, and opened the reaction system to continue the reaction in air for 24 h. Tracked by TLC until the reaction is complete, cool the reaction solution to room temperature, pour it into ice water, and a red solid precipitates out, filter it with suction to obtain the crude product, dry it in vacuum, and then separate and purify it by column chromatography, using dichloromethane as the eluent , ethyl acetate and ethanol volume ratio of 1:5 m...
Embodiment 2
[0044] Synthesis of Tuoxynone-Triphenylene Discotic Liquid Crystalline Compound (TRO-8-TP)
[0045]
[0046] Monobromotriphenylene TP (OC 6 h 13 ) 5 (OCH 2 ) 8 Br (131 mg, 0.14 mmol), potassium carbonate (96.6 mg, 0.70 mmol), and 15 mL N,N'-dimethylformamide were added to a 25 mL round bottom flask, stirred at room temperature, and the monobromotriphenylene Dissolved completely, under the protection of inert gas, added trihydroxytuximene compound (30 mg, 0.035 mmol), heated and stirred at 80°C, reacted for 40 h, then removed the protection, opened the reaction system and continued to react in air for 36 h. Tracked by TLC until the reaction is complete, cool the reaction solution to room temperature, pour it into ice water, and a red solid precipitates out, filter it with suction to obtain the crude product, dry it in vacuum, and then separate and purify it by column chromatography, using dichloromethane as the eluent , ethyl acetate and ethanol volume ratio of 1:5 mixe...
Embodiment 3
[0049] Synthesis of Tuoxynone-Triphenylene Discotic Liquid Crystalline Compound (TRO-10-TP)
[0050]
[0051] Monobromotriphenylene TP (OC 6 h 13 ) 5 (OCH 2 ) 10 Br (118 mg, 0.123 mmol), potassium carbonate (87 mg, 0.63 mmol), and 15 mL N,N'-dimethylformamide were added to a 25 mL round bottom flask, stirred at room temperature, and the monobromotriphenylene Dissolved completely, under the protection of inert gas, added trihydroxytuximene compound (30 mg, 0.035 mmol), heated and stirred at 80°C, reacted for 48 h, then removed the protection, opened the reaction system and continued to react in air for 32 h. Tracked by TLC until the reaction was complete, the reaction solution was cooled to room temperature, poured into ice water, and a red solid precipitated, and the crude product was obtained by suction filtration, dried in vacuo, and then separated and purified by column chromatography, dichloromethane was used as the eluent , ethyl acetate and ethanol volume ratio o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hole mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com