Method of synthesizing L-carnosine using L-amino acid ligase by one-step method
A technology of ligating enzymes and amino acids, applied in the biological field, can solve the problems of complex reaction products, difficult extraction and purification, etc., and achieve the effects of wide sources, easy large-scale production, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
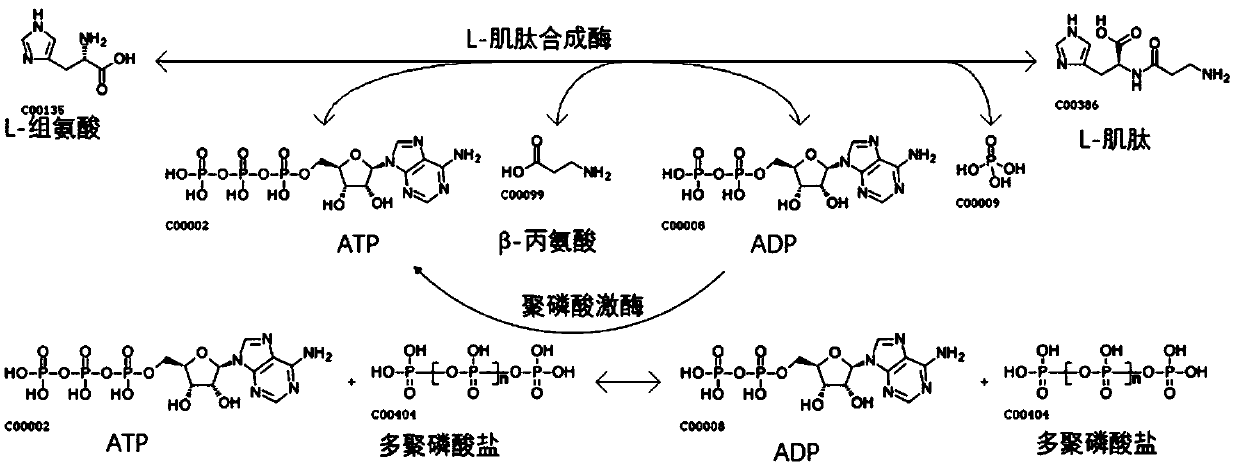
[0022] The present invention will be further described in detail below in conjunction with the accompanying drawings and specific embodiments.
[0023] The invention utilizes Escherichia coli to separately express L-amino acid ligase and polyphosphate kinase, which are purified and then mixed to form a dual-enzyme coupling system. L-amino acid ligase catalyzes the synthesis of L-carnosine from β-alanine and L-histidine, and at the same time, ATP is dephosphorylated to form ADP. Polyphosphate kinase catalyzes the transphosphorylation of polyphosphate to ADP to form ATP. In order to realize the recycling of ATP, such as figure 1 shown. Specific steps:
[0024] 1. Construction of recombinant L-amino acid ligase strain
[0025] 1.1: According to the L-amino acid ligase gene sequence whose GI number is GI: 1094636766, after codon optimization, an NdeI restriction site is added to the 5' end of the optimized sequence, and an XhoI restriction site is added to the 3' end to form SE...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com