Camptothecin prodrug gel, and preparation method and use thereof
A technology of camptothecin and prodrug, applied in the field of medicine, can solve the problems of limited application, low therapeutic effect, poor water solubility, etc., and achieves the effects of good glutathione response performance, broad clinical application prospect, and good pH response performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
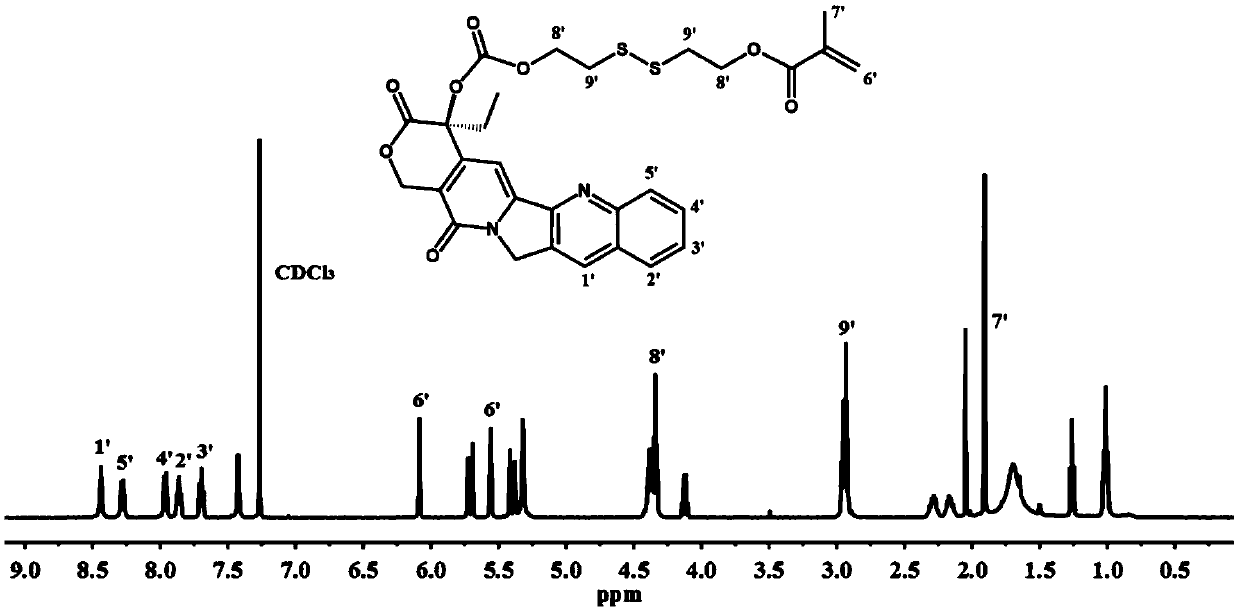
[0059] Example 1 Preparation of 2-((2-hydroxyethyl) disulfanyl) ethyl methacrylate (HSEMA)
[0060]
[0061] 2,2'-Dithiodiethanol (1.54g, 10mmol) and triethylamine (1.52g, 15mmol) were dissolved in 50ml of anhydrous tetrahydrofuran, and cooled to 0°C in an ice-water bath. Dissolve methacryloyl chloride (1.05, 10 mmol) in 25 mL of anhydrous tetrahydrofuran, and slowly add it dropwise to the above reaction solution under vigorous stirring. After reacting overnight at room temperature, insoluble salts were removed by filtration; then, all solvents were removed by rotary evaporation. The obtained crude product was diluted with 50 mL of ethyl acetate, and washed three times with water and saturated sodium chloride solution to remove impurities in unreacted raw materials. The organic phase was collected by separation and dried over anhydrous magnesium sulfate. The solution was concentrated by rotary evaporation, and then separated and purified by silica column, the mobile phase...
Embodiment 2
[0062] The preparation of embodiment 2CPT-ss-M
[0063]
[0064] Under a nitrogen atmosphere, camptothecin (0.70 g, 2 mmol) and triphosgene (0.2 g, 0.66 mmol) were blended in 50 mL of dry dichloromethane, followed by the addition of 4-dimethylaminopyridine (0.73 g, 6 mmol) , and stirred for 1 hour. Then HSEMA (0.55 g, 2.5 mmol) was dissolved in 10 ml of anhydrous THF, and added dropwise to the above reaction solution. After 24 hours of reaction at room temperature, the reaction mixture was filtered to remove insoluble salts; all solvent was removed by rotary evaporation. The residue was redissolved in dichloromethane, and washed twice with dilute hydrochloric acid (100 mmol / L), water, and saturated sodium chloride solution respectively. The organic layer was collected and dried over anhydrous magnesium sulfate. Concentrate the supernatant, use dichloromethane / methanol (200 / 1, v / v) as eluent, separate and purify by silica gel column, obtain light yellow camptothecin monom...
Embodiment 3
[0065] Example 3 Preparation of 2-(2-hydroxyethyl) ethyl methacrylate (HDOMA)
[0066]
[0067] 1,6-Hexanediol (1.18g, 10mmol) and triethylamine (1.52g, 15mmol) were dissolved in 50ml of anhydrous tetrahydrofuran, and cooled to 0°C in an ice-water bath. Dissolve methacryloyl chloride (1.05, 10 mmol) in 25 mL of anhydrous tetrahydrofuran, and slowly add it dropwise to the above reaction solution under vigorous stirring. After reacting overnight at room temperature, insoluble salts were removed by filtration; then, all solvents were removed by rotary evaporation. The obtained crude product was diluted with 50 mL of ethyl acetate, and washed three times with water and saturated sodium chloride solution to remove impurities in unreacted raw materials. The organic phase was collected by separation and dried over anhydrous magnesium sulfate. The solution was concentrated by rotary evaporation, then separated and purified by silica column, and the mobile phase was ethyl acetate / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com