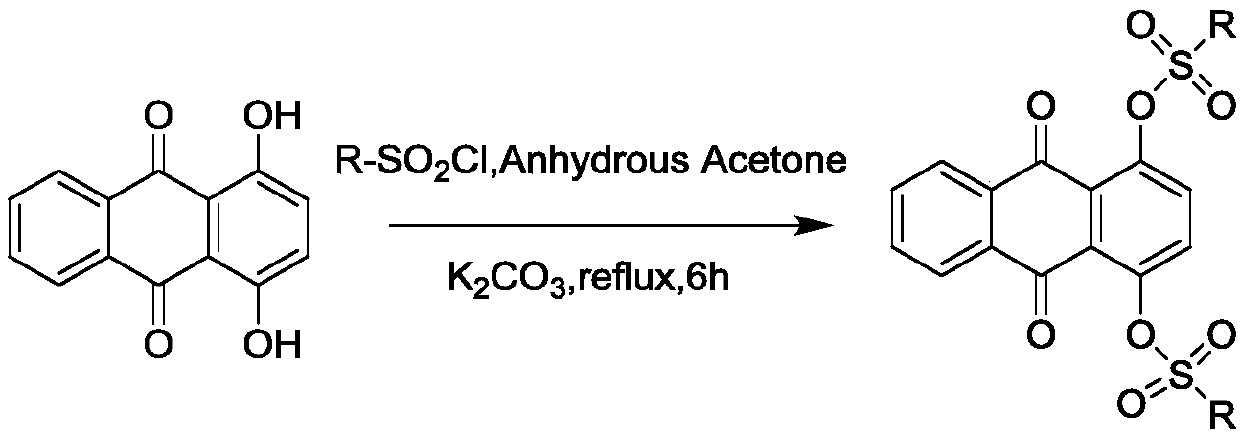
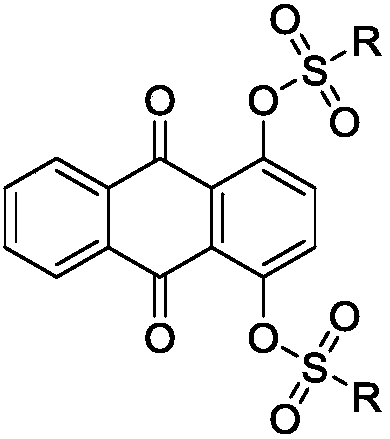
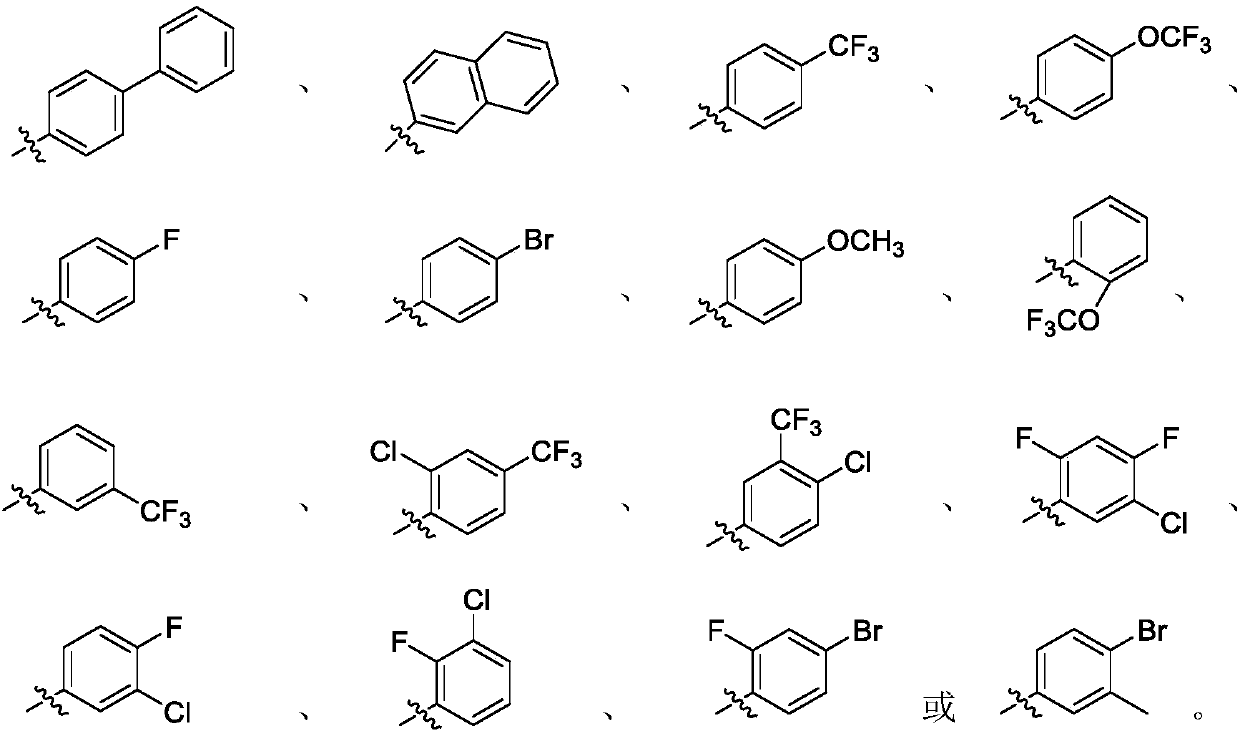
Sulfonyl ester group anthraquinone derivative, preparation method and application thereof
A technology of sulfonyl ester anthraquinones and derivatives, which is applied in the field of sulfonyl ester anthraquinone derivatives and their preparation methods and applications in pharmaceuticals, and can solve the problems that limit the wide application of anthracyclines, cardiomyocytes Toxicity and other problems, to achieve broad-spectrum anti-tumor activity, the effect of simple and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] 2. Preparation of sulfonyl ester anthraquinone derivatives The common raw material in the examples is 1,4-dihydroxy-9,10-anthraquinone, that is, 1,4-dihydroxyanthraquinone. The structure and atomic number of 1,4-dihydroxy-9,1-anthraquinone are as follows:
[0037]
[0038] Raw material chemical molecular structural formula (M01-M16) involved in the reaction process of table 2
[0039]
[0040]
Embodiment 1
[0041] The preparation of embodiment 1 sulfonyl ester group anthraquinone derivatives (C01-C16)
[0042] (1) Preparation of 1,4-bis(1,1'-biphenyl)-4-sulfonate)-9,10 anthraquinone (C01):
[0043] Add 0.5 g (2 mmol) of 1,4 dihydroxyanthraquinone, 1.2 g of excess 4-biphenylsulfonyl chloride, and 0.5 g of anhydrous potassium carbonate to a glass flask in sequence, then add 20 mL of anhydrous acetone, and react in an oil bath at 60°C Under reflux reaction, TLC monitors the reaction throughout the whole process. After the reaction is completed, add an appropriate amount of ice water to quench the reaction, filter to obtain a light yellow crude product, and purify it by silica gel column chromatography. Eluent: (dichloromethane:petroleum ether=1:2), The light yellow target compound was obtained with a yield of 74%.
[0044] (2) Preparation of 1,4-bis(2-naphthalenesulfonate)-9,10 anthraquinone (C02):
[0045] Add 0.5g (2mmol) of 1,4-dihydroxyanthraquinone, 1.4g of excess 2-naphthale...
Embodiment 2
[0074] The preparation of embodiment 2 sulfonyl ester group anthraquinone derivatives (C01-C16)
[0075] (1) Preparation of 1,4-bis(1,1'-biphenyl)-4-sulfonate)-9,10 anthraquinone (C01):
[0076] Add 0.1kg of 1,4-dihydroxyanthraquinone, 0.5kg of excess 4-biphenylsulfonyl chloride raw material, 0.1kg of anhydrous potassium carbonate, and then add 4L of anhydrous acetone in a stainless steel reaction kettle, and react in an oil bath at 60°C Reflux reaction, TLC monitors the reaction throughout the whole process, after the reaction is complete, add an appropriate amount of ice water to quench the reaction, filter to obtain a light yellow crude product, purify by silica gel column chromatography, eluent (dichloromethane:petroleum ether=1:2), and obtain pale yellow product Yellow target compound, yield 65%.
[0077] (2) Preparation of 1,4-bis(2-naphthalenesulfonate)-9,10 anthraquinone (C02):
[0078] Add 0.1kg (0.4mol) of 1,4-dihydroxyanthraquinone, 0.5kg of excess 2-naphthalenesu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com