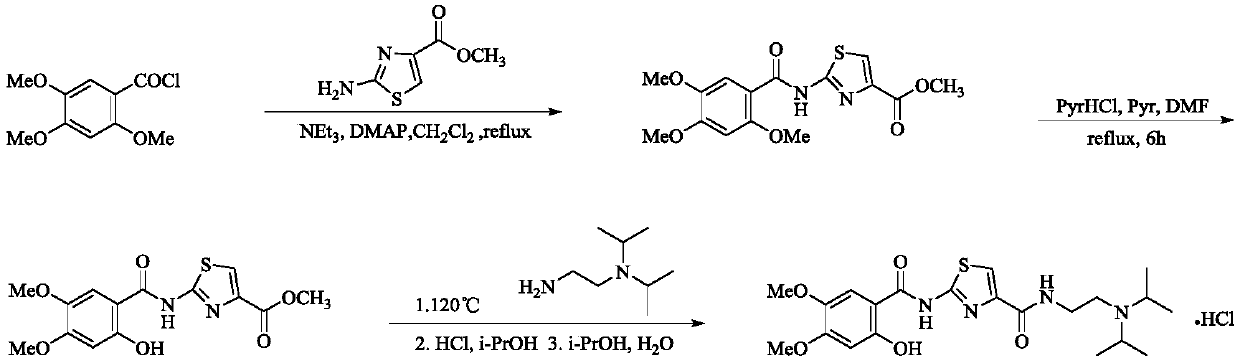
Improved synthesis method for hydrochloric acid acotiamide
A technology of acotiamide hydrochloride and a synthesis method, which is applied in the field of synthesis of acotiamide hydrochloride, can solve the problems of complicated post-processing, high production cost, low conversion rate of raw materials, etc., and achieves stable product quality, low price, and purity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
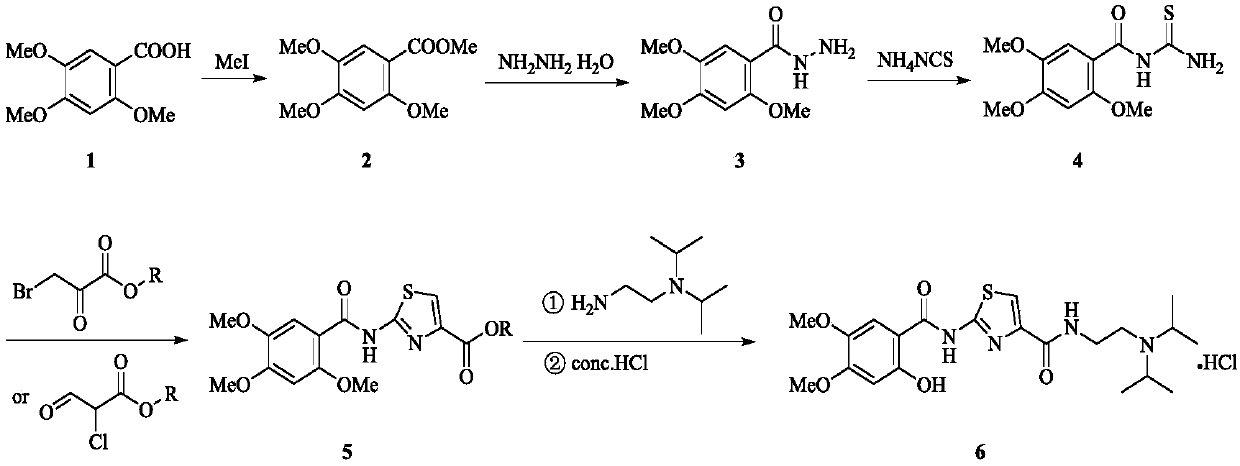
[0027] (1) Add 2,4,5-trimethoxybenzoic acid (106g, 0.5mol) and sodium hydroxide (40g, 1mol) to 1500mL of methanol, stir for half an hour, and slowly add 62.3mL of methyl iodide dropwise at room temperature (1mol), the dropwise addition was completed, the system was heated to 50° C., and reacted for 5 hours, and the reaction was completed. The system was cooled to room temperature, concentrated under reduced pressure, added 2000 mL of water, and extracted with ethyl acetate (1500 mL×3). The organic phases were combined, washed with water (2000 mL×3), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain intermediate 2 with a yield of 99%.
[0028] (2) Intermediate 2 (22.6g, 0.1mol) and 250mL of methanol solvent were placed in a 500mL three-necked flask, and 80% by mass hydrazine hydrate (9.4g, 0.15mol) was slowly added at room temperature, heated to reflux, The reaction was stirred for 6 hours, and the reaction was completed. The sys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com