Uterine cavity compression hemostatic balloon catheter and control system thereof
A balloon catheter, compression hemostasis technology, applied in the direction of balloon catheters, catheters, other medical devices, etc., can solve the problems of water bladder dead angle, difficult uterine cavity hemostatic effect, unable to adjust, etc., to achieve good biocompatibility, increase Intrauterine infection, easy to adjust the effect of positioning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
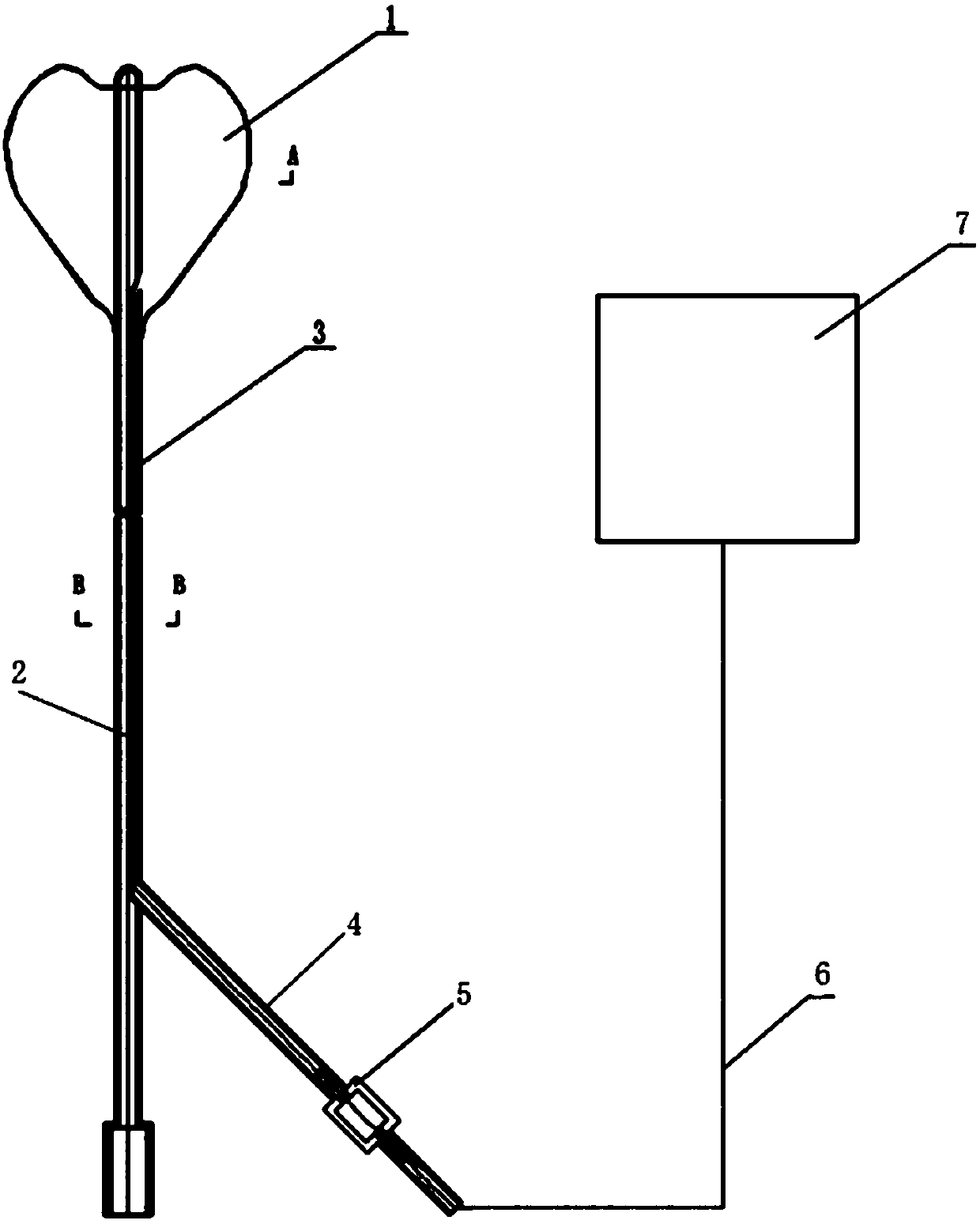
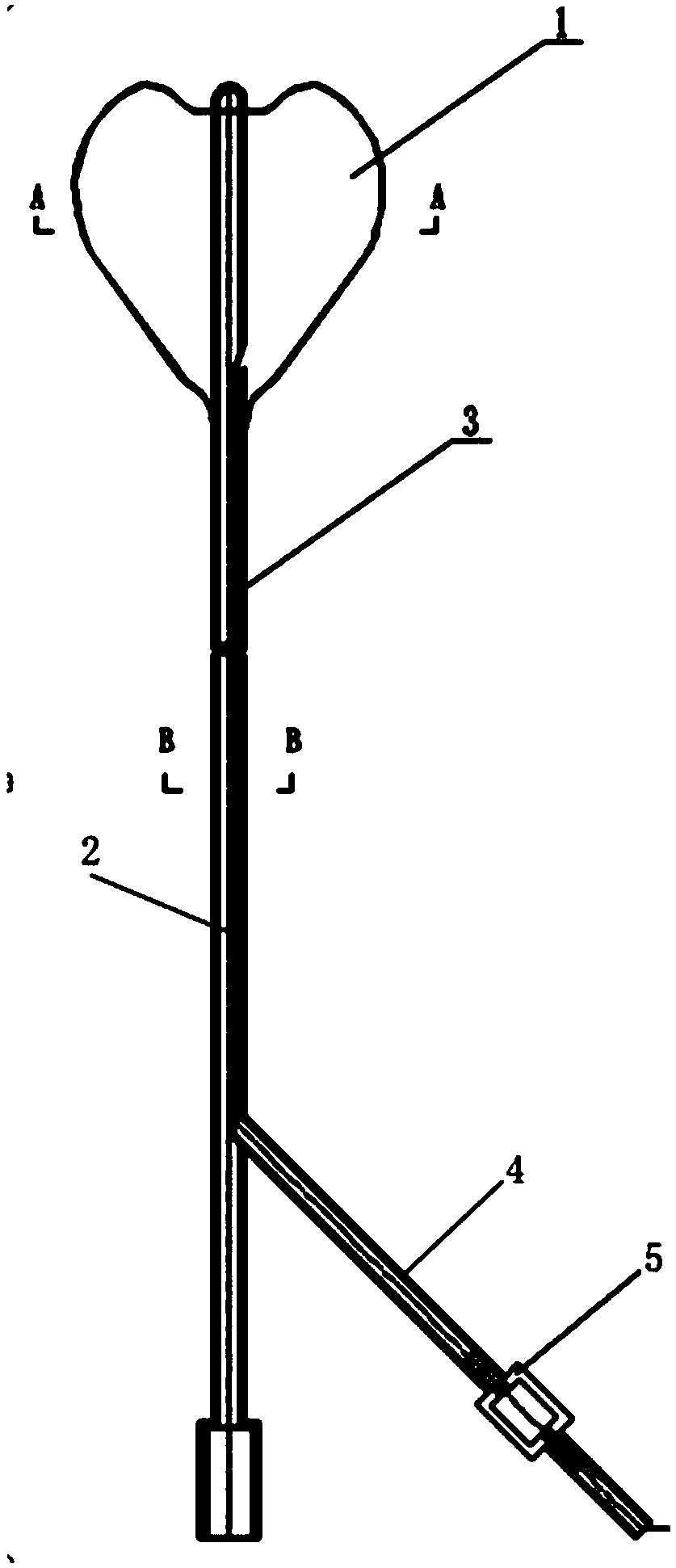
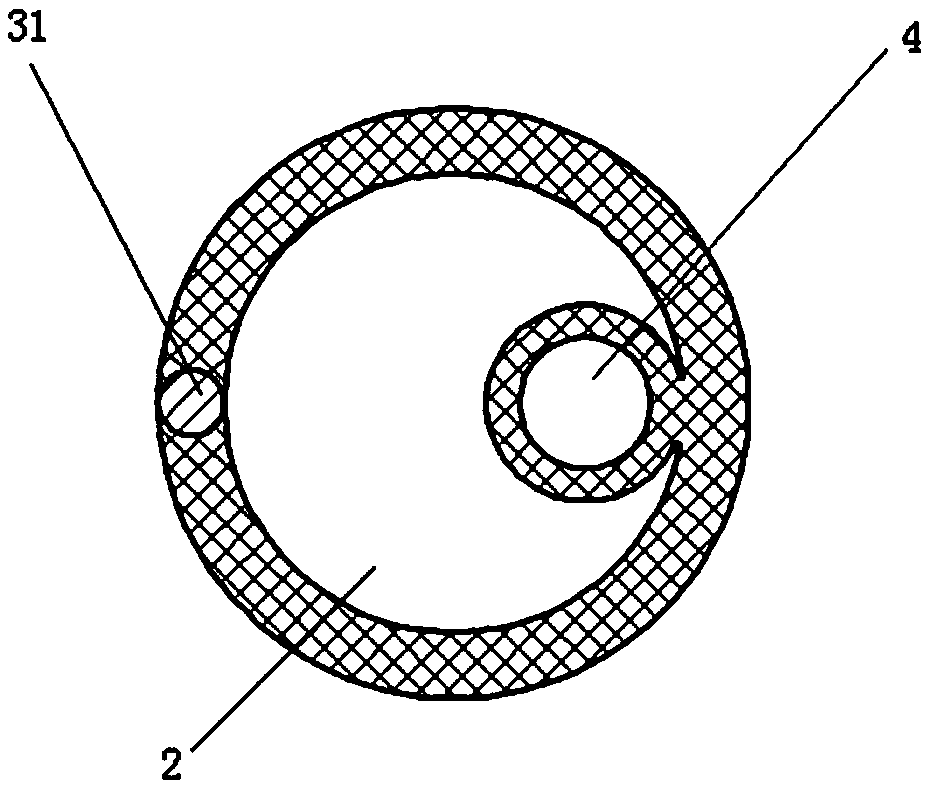
[0045] In this example, the intrauterine compression hemostatic balloon catheter is made of silicone rubber; the wall of the balloon catheter is provided with an X-ray-opaque contrast strip along the longitudinal direction of the tube body, and the section of the balloon catheter is as follows: image 3 As shown, it includes a liquid injection channel, a liquid drainage channel and a contrast strip 31 .
[0046] The contrast strip is made of a blended material of silicone rubber and barium sulfate, and the silicone rubber can adopt a peroxide vulcanization system or an addition vulcanization system.
[0047] Balloon catheter inner diameter: 3-6mm.
[0048] Balloon catheter wall thickness: 0.5-2mm.
[0049] Catheter material formulation: silicone rubber: 100 (HCRA4150A / B, Elkem Silicones)
[0050] Silicone rubber: 100 (HCRA4170A / B, Elkem Silicones)
[0051] Catheter contrast strip formula: silicone rubber: 100 (HCRA4150A / B, Elkem Silicones)
...
Embodiment 2
[0062] Balloon catheter inner diameter: 3-6mm
[0063] Balloon catheter wall thickness: 0.5-2mm
[0064] Balloon catheter material formula: silicone rubber: 100 (C6-150A / B, DOW CORNING company)
[0065] Silicone rubber: 100 (C6-170A / B, DOW CORNING company)
[0066] Balloon Catheter Contrast Strip Formula: Silicone Rubber: 100 (C6-150A / B, DOW CORNING Company)
[0068] Balloon thickness: 0.3-0.6mm
[0069] Balloon material formula: silicone rubber: 100 (Q7-4720A: DOW CORNING company)
[0070] Silicone rubber: 100 (Q7-4720B: DOW CORNING company)
[0071] Balloon contrast strip formula: silicone rubber: 100 (Q7-4720A / B: Elkem Silicones)
[0072] Barium sulfate: 15-30
Embodiment 3
[0074] Balloon catheter inner diameter: 3-6mm
[0075] Balloon catheter wall thickness: 0.5-2mm
[0076] Balloon catheter material formula: silicone rubber: 50 (Silpuran8030 / 50WACKER company)
[0077] Silicone rubber: 50 (Silpuran8030 / 70WACKER company)
[0078] Vulcanizing agent: 1.0-2.0
[0079] Balloon catheter angiography strip formula: silicone rubber: 100 (Silpuran8030 / 50WACKER company)
[0080] Vulcanizing agent: 1.0-2.0
[0081] Barium sulfate: 10-30
[0082] Balloon thickness: 0.3-0.6mm
[0083] Balloon material formula: silicone rubber: 100 (Silpuran8030 / 30WACKER company)
[0084] Vulcanizing agent: 1.0-2.0
[0085] Balloon contrast strip formula: silicone rubber: 100 (Silpuran8030 / 30WACKER company)
[0086] Vulcanizing agent: 1.0-2.0
[0087] Barium sulfate: 15-30
[0088] It should be noted that the formulation can be uniformly used in units of measurement such as grams, kilograms, and liters.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com