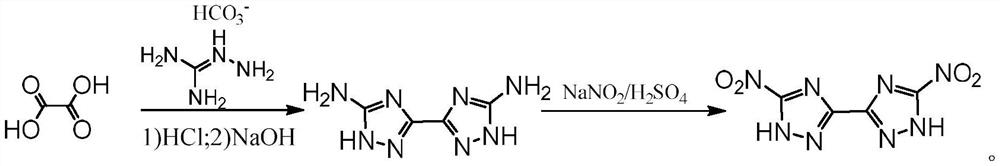
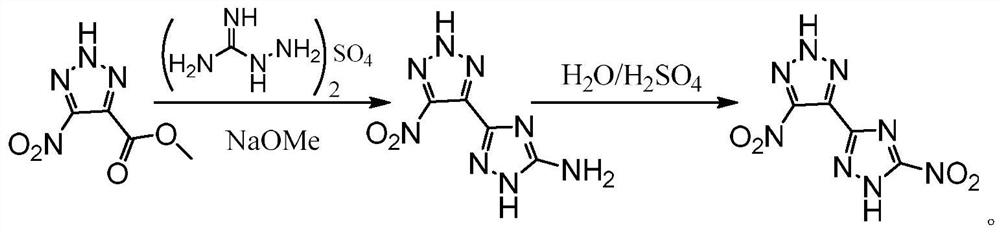
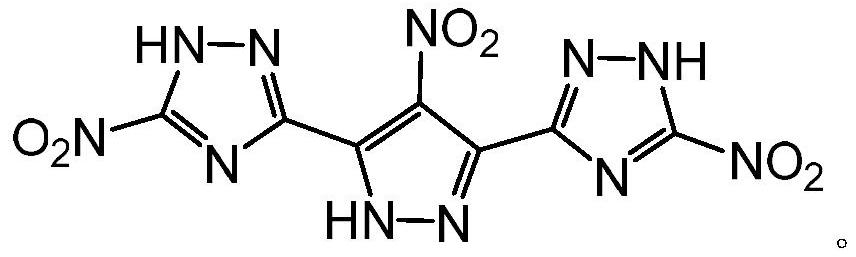
A kind of high-energy insensitive energetic compound and its synthesis method
A synthesis method and compound technology, applied in the direction of nitrated acyclic/alicyclic/heterocyclic amine explosive composition, organic chemistry, etc., can solve the problems of high toxicity, complicated preparation steps, safety and environmental protection, etc. Achieve the effect of good thermal stability and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation steps of target product of the present invention are as follows:
[0032] Ⅰ. Add metal sodium to the anhydrous methanol solution several times at 0°C to prepare the sodium methoxide solution, then slowly add aminoguanidine sulfate in batches, and finally add 4-nitro-1H-pyrazole-3,5- Dimethyl diformate (1) was added to the mixed solution several times, after the addition, the temperature was raised to reflux for reaction, after the reaction was finished, it was cooled and filtered, and the filter cake was washed several times with methanol solution, the combined filtrate was concentrated under reduced pressure and poured into ice water. Adjust the pH to about 5 to have solid precipitation, filter, wash, and dry to obtain orange solid 4-nitro-3,5-bis(1H-1,2,4-triazole-3-amino)-1H-pyrazole ( 2), wherein the molar ratio of sodium metal:aminoguanidine sulfate:4-nitro-1H-pyrazole-3,5-dicarboxylic acid dimethyl ester=5:5:1;
[0033] Ⅱ. At 0°C, slowly add 4-nit...
Embodiment 1
[0035] a) Synthesis of intermediate 4-nitro-3,5-bis(1H-1,2,4-triazole-3-amino)-1H-pyrazole
[0036] Sodium metal (1.20 g, 52.2 mmol) was dissolved in 50 mL of methanol at 0° C., and aminoguanidine sulfate (12.20 g, 50.0 mmol) was slowly added thereto in several portions. After the addition, take dimethyl 4-nitro-1H-pyrazole-3,5-dicarboxylate (2.30g, 10.0mmol) and slowly add it to the solution, heat to reflux for 24-48h, cool to room temperature, filter, and wash with methanol Wash the filter cake several times, combine the filtrate, concentrate under reduced pressure to 20 mL, add 50 mL of ice water to it, add concentrated HCl dropwise to adjust the pH to about 5, filter, and dry to obtain orange-yellow solid 4-nitro-3,5-bis (1H-1,2,4-triazole-3-amino)-1H-pyrazole 2.36g, yield 85.2%.
[0037] Attachment: Intermediate decomposition temperature: 353.6°C; 1 H NMR (300MHz, DMSO-d 6 ): δ=6.26(s,4H), 3.59(br)ppm; 13 C NMR (125MHz, DMSO-d 6 ): δ=161,155,147,137ppm; mass spectrum...
Embodiment 2
[0044] At 0° C., sodium metal (1.40 g, 60.8 mmol) was dissolved in 50 mL of methanol, and aminoguanidine sulfate (15.00 g, 61.2 mmol) was slowly added thereto in several portions. After the addition, dimethyl 4-nitro-1H-pyrazole-3,5-dicarboxylate (2.30g, 100.0mmol) was slowly added to the solution, heated to reflux for 24-48h, cooled to room temperature, filtered, and washed with methanol Wash the filter cake several times, combine the filtrate, concentrate under reduced pressure to 20 mL, add 50 mL of ice water to it, add concentrated HCl dropwise to adjust the pH to about 5, filter, and dry to obtain orange-yellow solid 4-nitro-3,5-bis (1H-1,2,4-triazole-3-amino)-1H-pyrazole 2.25 g, yield 81.2%.
[0045] Take 20mL30%H 2 o 2 Put it into a 50mL three-necked bottle, set the low temperature bath to -20°C, and slowly add 15mL of 98% H 2 SO 4 , the temperature is controlled below 0°C during the dropwise addition. After the dropwise addition is completed, add 4-nitro-3,5-bis(1H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com