Composition, differentiation inducing culturing liquid containing composition and inducing method
A technology of differentiation induction and composition, applied in the field of cells, can solve the problems of lack of mechanical properties and durability of hyaline cartilage, and unsatisfactory results, and achieve the effects of low immunogenicity, promotion of repair and regeneration, and easy expansion in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
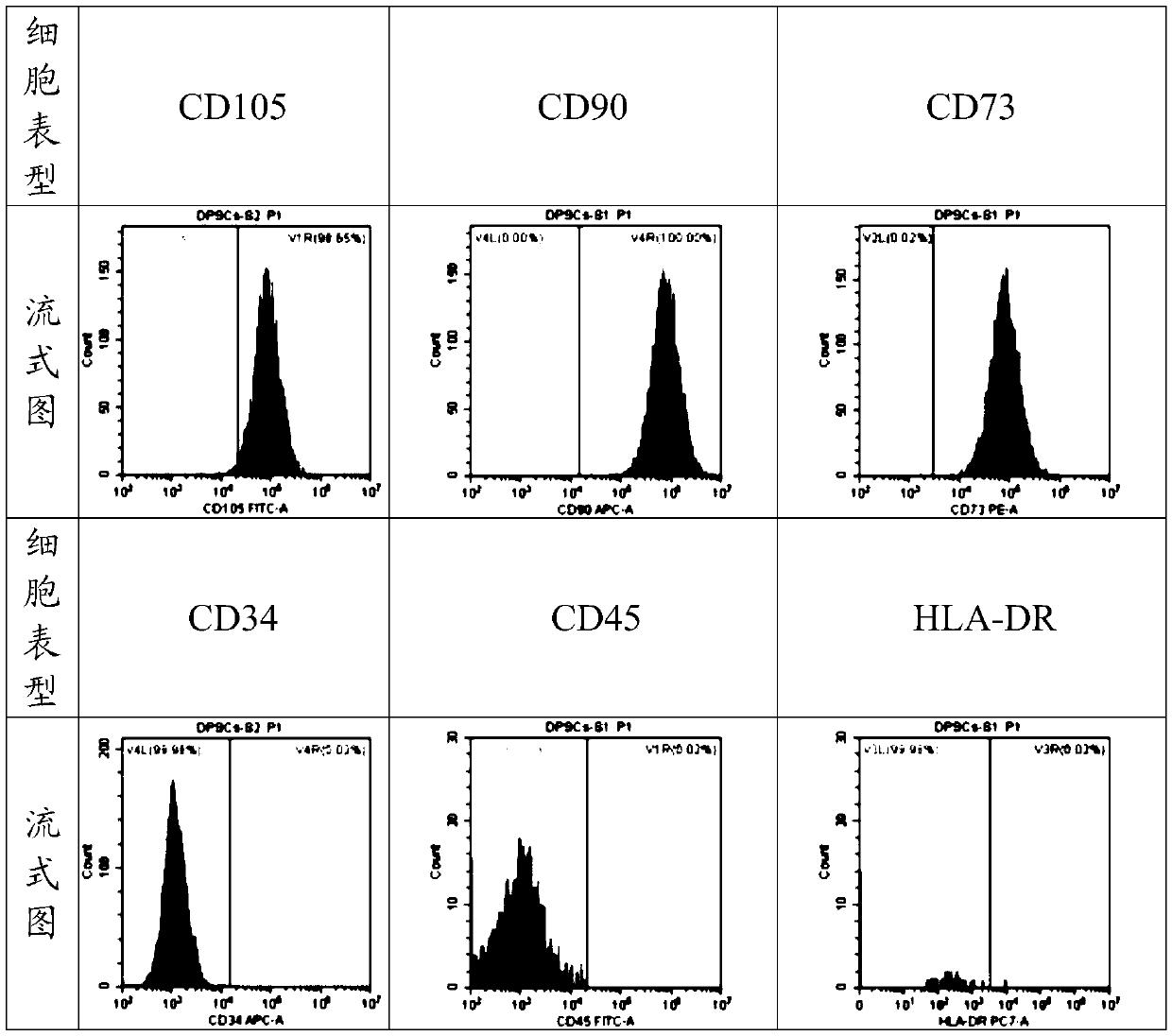
[0034] Example 1 Primary isolation culture, identification and passage of DPSCs
[0035] Samples: Complete premolars or third molars extracted for orthodontics from 10-20-year-old healthy donors were quickly immersed in 4°C pre-cooled PBS containing 3 times double antibody.
[0036] Rinse: Rinse the teeth with PBS containing 3 times the double antibody to remove blood stains thoroughly. Repeat 3 times.
[0037] Pulp separation and extraction: Wrap the tooth in sterile gauze and crack the tooth with forceps to expose the pulp tissue; use sterile forceps to grasp the pulp tissue, and remove the pulp tissue at the root tip of 1 mm. Cut the pulp tissue to 1mm with ophthalmic curved scissors 3 Left and right size tissue blocks.
[0038] Digestion: Add 5-20 times the volume of collagenase-dispase enzyme 1:1 mixed digestion solution (collagenase 3g / L, dispase enzyme 4g / L), and place it in a constant temperature shaker at 37°C for 45-50min. After digestion, centrifuge at 1500r / min...
Embodiment 2
[0057] Take the 3rd generation DPSCs, press 5×10 5 The cell / tube was inoculated in a 15mL centrifuge tube, centrifuged at 1000rpm for 5min, so that the cells gathered at the bottom of the centrifuge tube, loosen half of the cap, and placed in 37°C, 5% CO 2 Culture in an incubator.
[0058] Discard the old solution after 24 hours, and replace with 1 mL of the experimental composition of chondrogenic induction solution: containing 10 μg / L TGF-β3, 50 μg / L proline, 5% PRP, 1% ITS + Premix high glucose DMEM medium. Gently flick the centrifuge tube to float the cell mass, loosen half of the tube cap, and place at 37°C, 5% CO 2 Culture in an incubator.
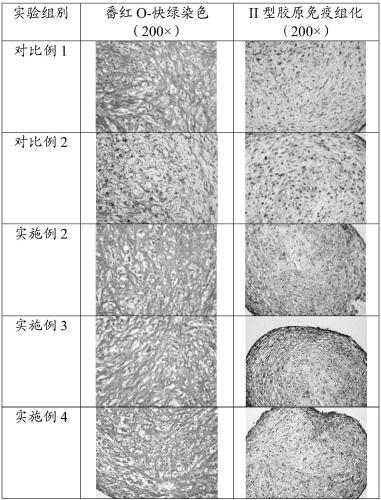
[0059] After 21 days of induced differentiation, the cells were subjected to paraffin section, safranin O-fast green staining and type II collagen immunohistochemistry experiments.
[0060] Safranin O-fast green staining experiment results are as follows image 3 As shown, the cell section is colored, and the colored area is obv...
Embodiment 3
[0062] Take the 3rd generation DPSCs, press 5×10 5 The cell / tube was inoculated in a 15mL centrifuge tube, centrifuged at 1000rpm for 5min, so that the cells gathered at the bottom of the centrifuge tube, loosen half of the cap, and placed in 37°C, 5% CO 2 Culture in an incubator.
[0063] Discard the old solution after 24 hours, and replace with 1 mL of the experimental composition of chondrogenic induction solution: containing 100 μg / L TGF-β3, 10 μg / L proline, 1% PRP, 5% ITS + Premix high glucose DMEM medium. Gently flick the centrifuge tube to float the cell mass, loosen half of the tube cap, and place at 37°C, 5% CO 2 Culture in an incubator.
[0064] After 21 days of induced differentiation, the cells were subjected to paraffin section, safranin O-fast green staining and type II collagen immunohistochemistry experiments.
[0065] Safranin O-fast green staining experiment results are as follows image 3 As shown, the cell section is colored, and the colored area is ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com