Mesenchymal stem cell for treating autoimmune diseases and preparation method and application of mesenchymal stem cell
A technology for autoimmune diseases and mesenchymal stem cells, applied in mesenchymal stem cells and its preparation method and application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: IL17RA-ECD: Fc fusion protein gene vector construction
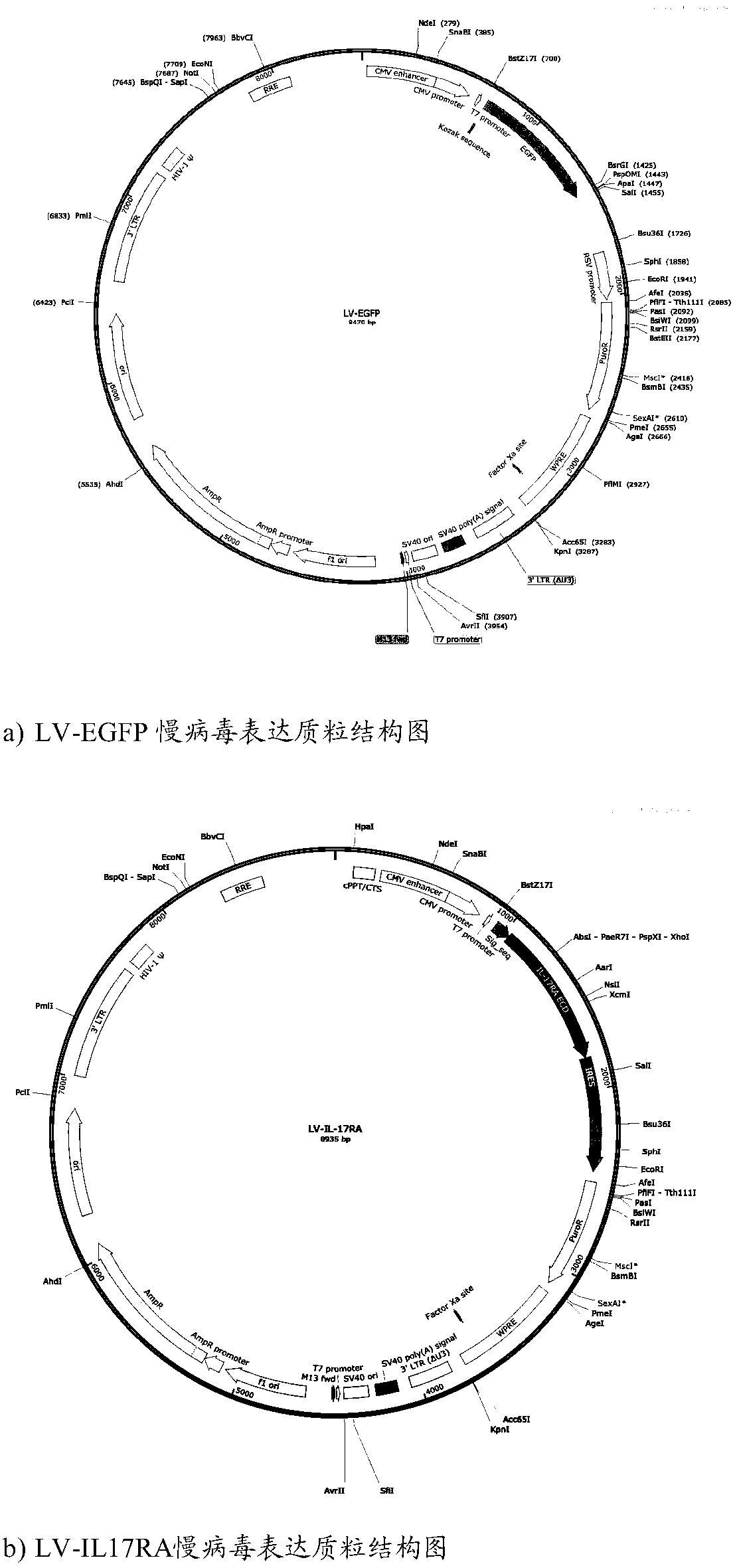
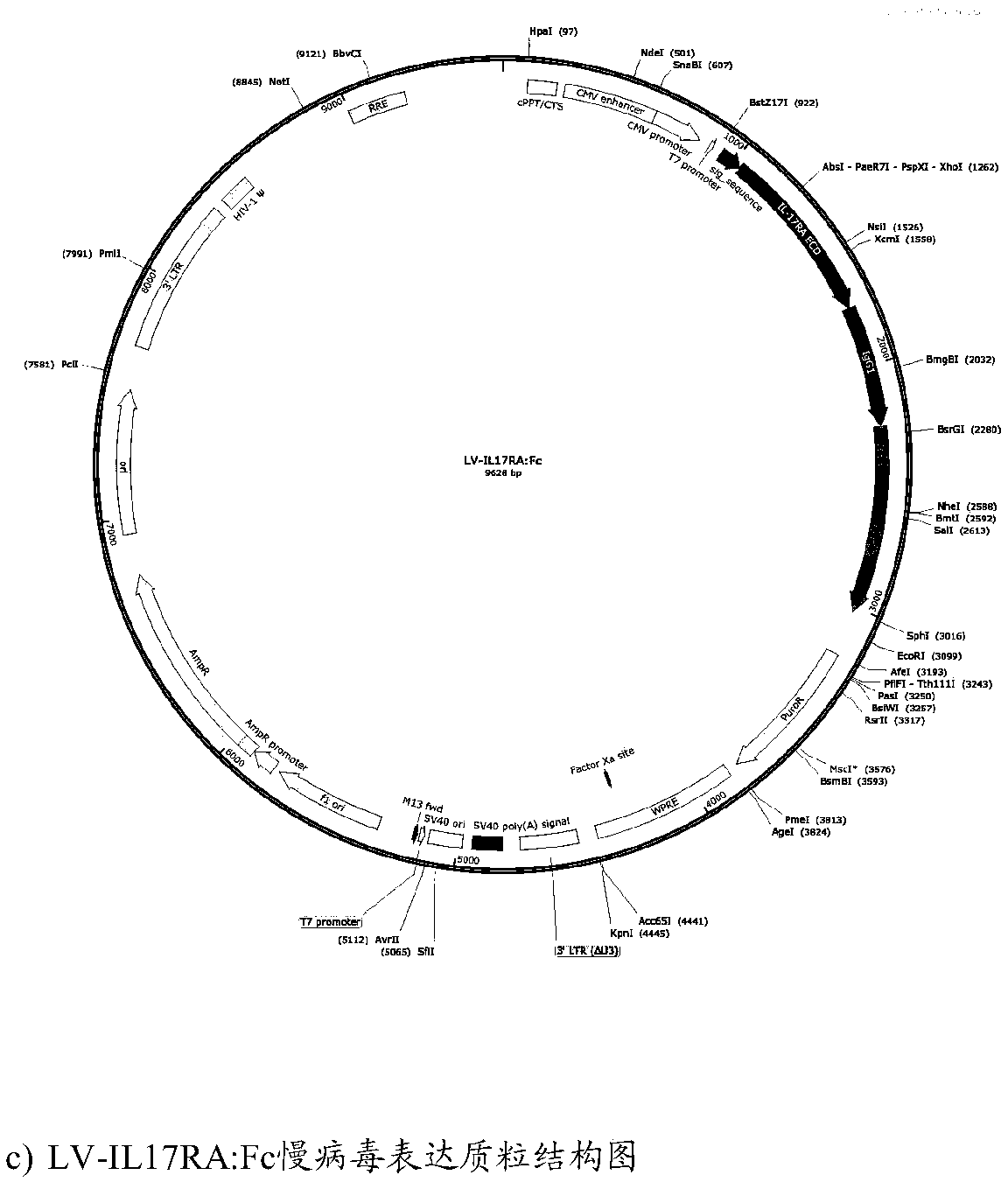
[0065] We designed IL17RA-ECD and IL17RA-ECD:Fc gene plasmids through Genebank, and constructed IL17RA-ECD and IL17RA-ECD:Fc fusion protein lentiviral expression plasmids using the fourth-generation lentiviral vector system (LV-IL17RA and LV-IL17RA:Fc ), and LV-EGFP was used as a control virus (LV-null). ( figure 1 Lentiviral expression plasmid constructs for LV-EGFP, LV-IL17RA and LV-IL17RA-Fc).
Embodiment 2
[0066] Example 2: Establishment of human umbilical cord mesenchymal stem cell seed bank
[0067] Human umbilical cords were obtained from healthy full-term pregnancy cesarean section pregnant woman donors, provided by qualified national second-level hospitals, and pregnant women or their family members signed informed consent and umbilical cord collection registration forms. The extraction, cultivation and establishment of cell bank of umbilical cord mesenchymal stem cells are all completed in our company's GMP production workshop. After the cells are separated, cultured, subcultured and expanded to the second generation (P2), the exogenous microorganisms, viruses, endotoxins, etc. are detected; at the same time, the immune phenotype, differentiation ability and cell biological efficacy of the cells are detected. Qualified cells were used as seed bank cells and stored in a liquid nitrogen tank at -196°C.
Embodiment 3
[0068] Example 3: IL17RA-ECD: Fc Gene Modified Mesenchymal Stem Cells
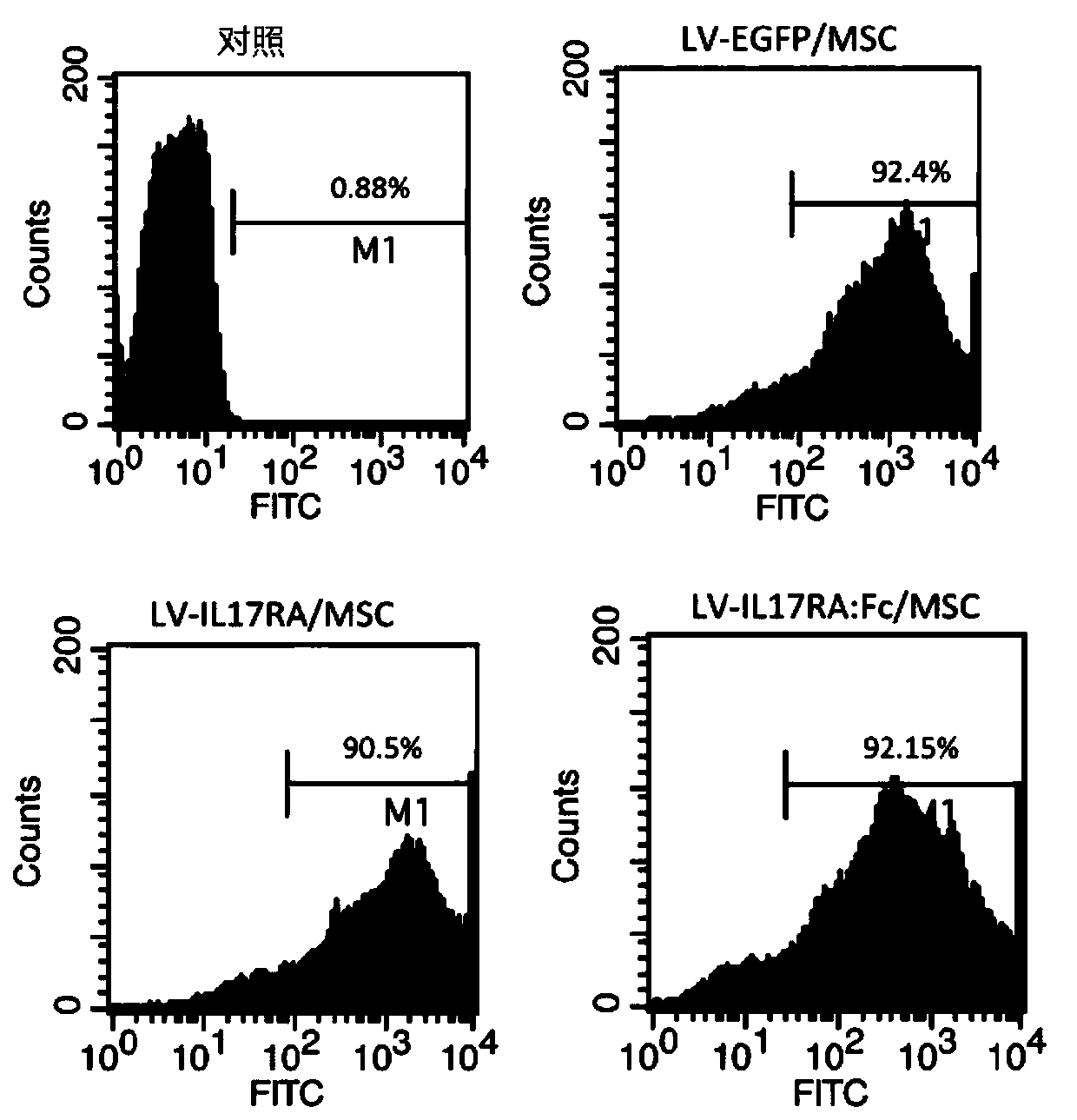
[0069] The above-mentioned LV-IL17RA and LV-IL17RA: Fc fusion protein lentiviral expression plasmids and LV-EGFP were mixed with lentiviral pGag / Pol, pRev, pVSV-G and other framework plasmids respectively, introduced into 293T cells through LTX liposomes, and packaged to obtain Mature lentiviruses were harvested and infected with MSCs. After 24 hours, puromycin was added to select successfully infected cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com