Low-cost, simple and rapid glycoprotein N-carbohydrate chain analysis method
A low-cost technology for glycoproteins, applied in the field of biochemistry, can solve the problems of weak N-glycan mass spectrometry signals and few types of sugar chains, and achieve the effects of increasing analytical abundance, simple operation, and stable and reliable analysis results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
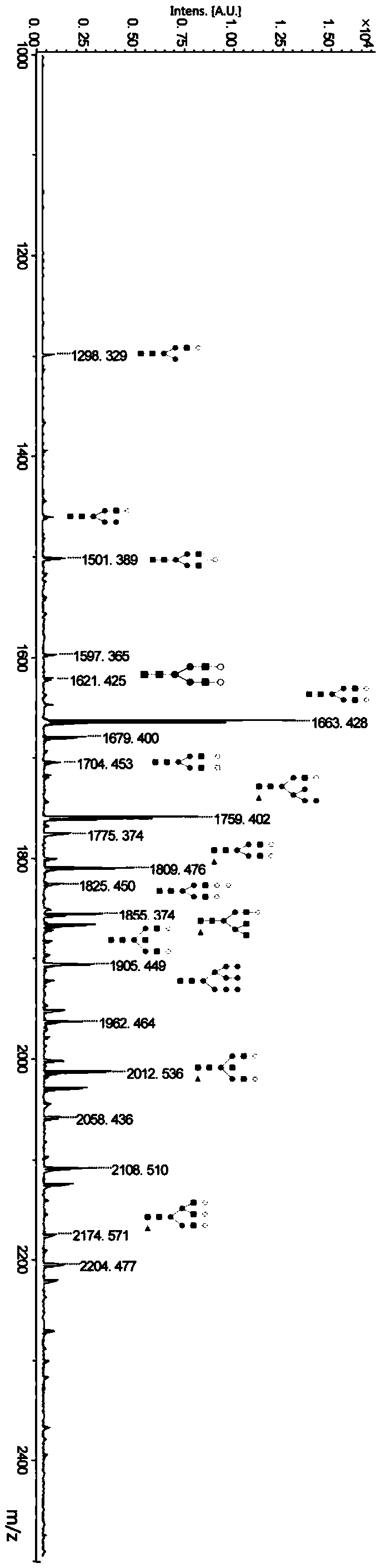
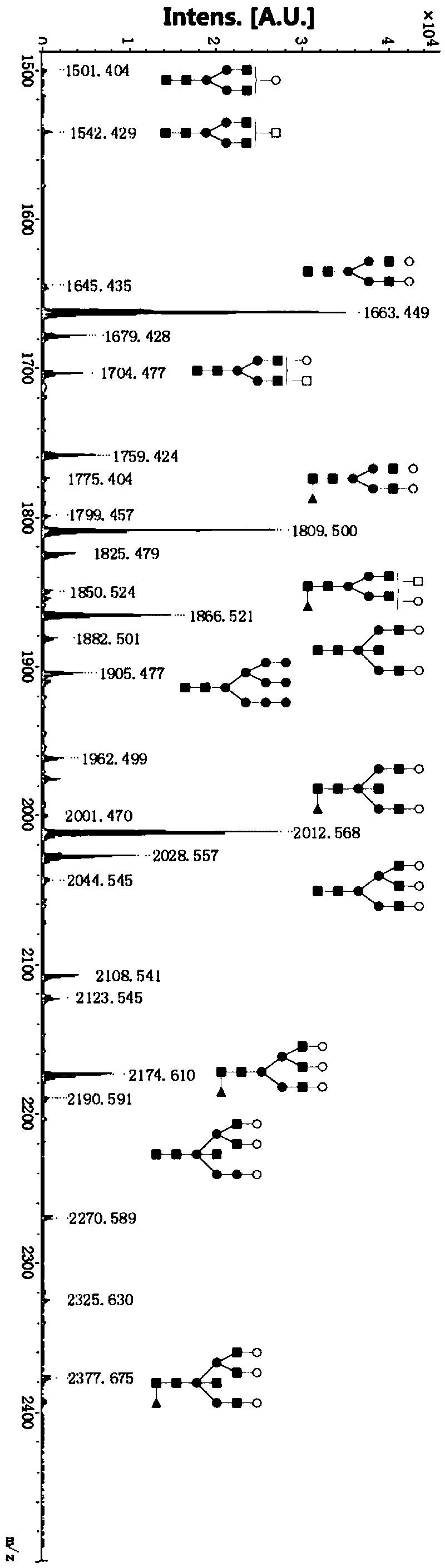
[0037] Example 1 N-glycan analysis of glycoprotein immunoglobulin A1 (IgA1) in human blood
[0038] 1. Method
[0039] (1) 100 μg of IgA1 obtained by separation and purification from human blood was dissolved in 200 μL of 50 mmol / L ammonium bicarbonate (NH 4 HCO 3 ) solution, and denatured by heating at 100°C for 10 min.
[0040] (2) Dithiothreitol (DTT) at a final concentration of 20 mmol / L was added, shaken at 56°C for 45 min, and iodoacetamide (IAM) at a final concentration of 50 mmol / L was added and reacted at room temperature for 1 h in the dark.
[0041] (3) All the reaction solution was added to a 30KD ultrafiltration tube, centrifuged at 13000g for 15min×3 times, each time with 200μL of 50mmol / L NH 4 HCO 3 rinse.
[0042] (4) Add 100 μL of 50 mmol / L NH 4 HCO 3 and 5U (1:20) PNGase F protease solution, and continue to react in a shaker at 37°C for 2h.
[0043] (5) Centrifuge at 13,000 g for 15 min x 3 times to collect N-glycan chains, and wash each time with 200...
Embodiment 2
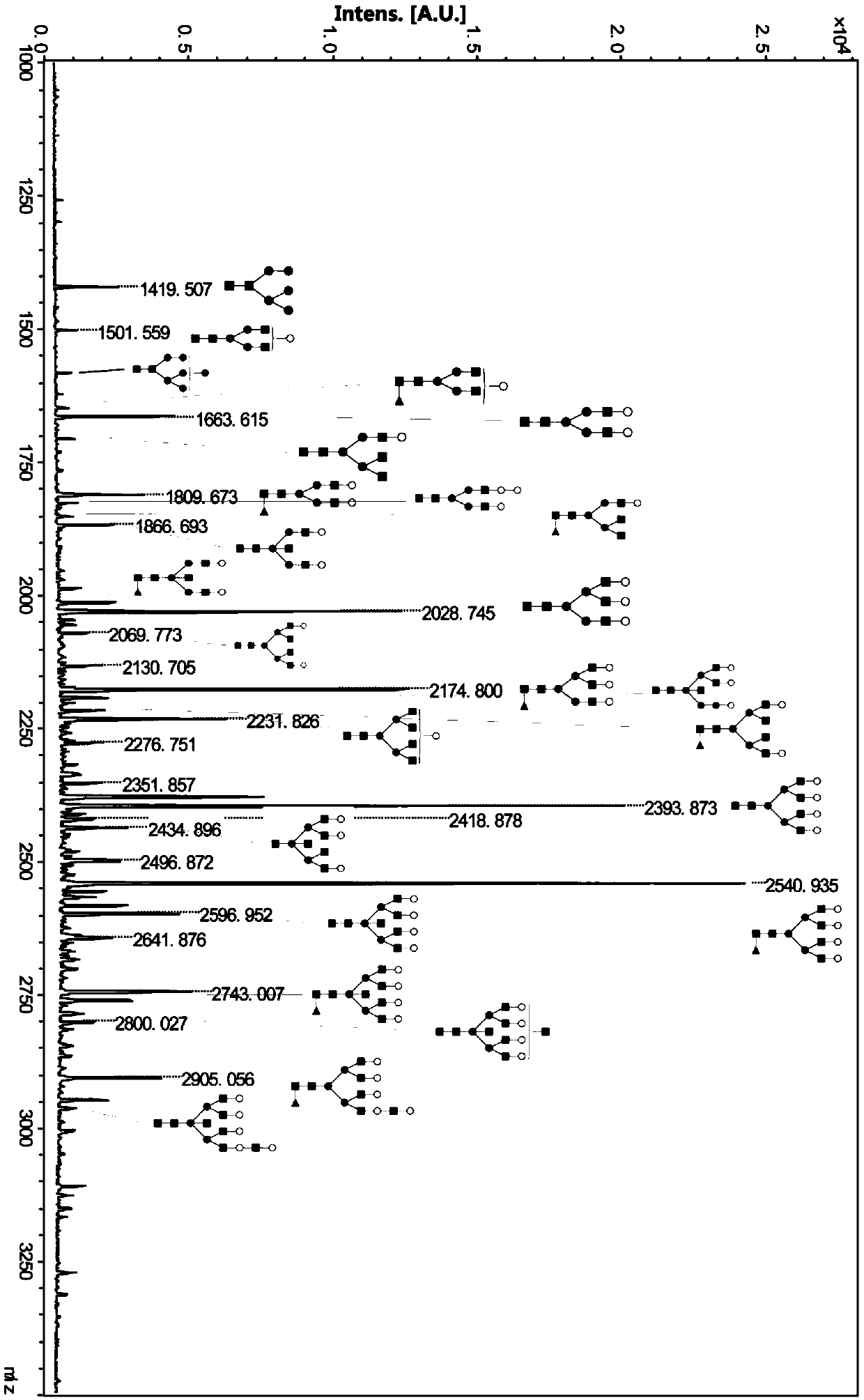
[0055] Example 2 N-glycan analysis of glycoprotein uromodulin (UMOD) in human urine
[0056] 1. Method
[0057] (1) Dissolve 100 μg of UMOD obtained by separating and purifying human urine into 200 μL of 50 mmol / L ammonium bicarbonate (NH4HCO3) solution, and heat at 100° C. for 10 minutes for denaturation.
[0058] (2) Dithiothreitol (DTT) at a final concentration of 20 mmol / L was added, shaken at 56°C for 45 min, and iodoacetamide (IAM) at a final concentration of 50 mmol / L was added and reacted at room temperature for 1 h in the dark.
[0059] (3) All the reaction solution was added to a 30KD ultrafiltration tube, centrifuged at 13000 g for 15 min x 3 times, and washed with 200 μL of 50 mmol / L NH4HCO3 each time.
[0060] (4) Add 100 μL of 50 mmol / L NH4HCO3 and 5U (1:20) PNGaseF protease solution, and continue to react for 2 h in a shaker at 37°C.
[0061] (5) Centrifuge at 13,000 g for 15 min x 3 times to collect N-glycan chains, and wash each time with 200 μL of ultrapure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com