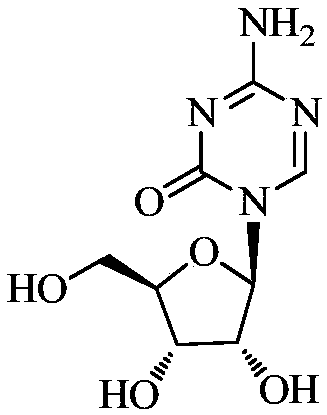
Stable azacitidine freeze-drying preparation and preparing method thereof
An azacitidine and freeze-drying technology, which is applied in freeze-dried delivery, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. Glycoside hydrolysis and other problems, to achieve the effect of accelerating the dissolution rate, reducing the crystal size and reducing the degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
Add to 2400ml (80%)
[0032] Preparation:
[0033] Measure 80% of water for injection, add the prescribed amount of mannitol into the water for injection, stir and dissolve, and cool down to 0-4°C, add the prescribed amount of azacitidine into tert-butanol and stir to disperse; add the mannitol aqueous solution Mix with azacitidine tert-butanol dispersion, and add the remaining amount of water for injection to obtain an intermediate liquid, keep the intermediate liquid at 0-4°C; filter the intermediate liquid through nitrogen pressure to 0.2 μm Filter the membrane, and sub-pack 24ml into injection bottles, freeze-dry, stopper, crimp the cap, pack, and obtain the azacitidine freeze-dried preparation. The freeze-drying process is as follows: Pre-freezing: keep at -45°C for 1.5h, -10°C for 1h, and -45°C for 2h; primary drying: keep at -10°C for 20h; secondary drying: keep at 40°C for 10h.
Embodiment 2
Add to 2400ml (80%)
[0036] Preparation:
[0037] Measure 80% of water for injection, add the prescribed amount of mannitol into the water for injection, stir and dissolve, and cool down to 0-4°C, add the prescribed amount of azacitidine into propylene glycol and stir to disperse; Zacytidine propylene glycol dispersions were mixed, and the remaining amount of water for injection was added to obtain an intermediate medicinal solution, which was kept at 0-4°C; the intermediate medicinal solution was filtered through a 0.2 μm filter membrane under nitrogen pressure, and Pack 24ml into injection vials, freeze-dry, cork, cap, and pack to obtain the azacitidine freeze-dried preparation. The freeze-drying process is the same as in Example 1.
Embodiment 3
Add to 2400ml (80%)
[0040] Preparation:
[0041] Measure 80% of water for injection, add the prescribed amount of mannitol into the water for injection, stir and dissolve, and cool down to 0-4°C, add the prescribed amount of azacitidine into isopropanol and stir to disperse; add the mannitol aqueous solution Mix with azacitidine isopropanol dispersion, and add the remaining amount of water for injection to obtain the intermediate drug solution, keep the intermediate drug solution at 0-4°C; filter the intermediate drug solution through nitrogen pressure to 0.2 μm Filter the membrane, and sub-pack 24ml into injection bottles, freeze-dry, stopper, crimp the cap, pack, and obtain the azacitidine freeze-dried preparation. The freeze-drying process is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com