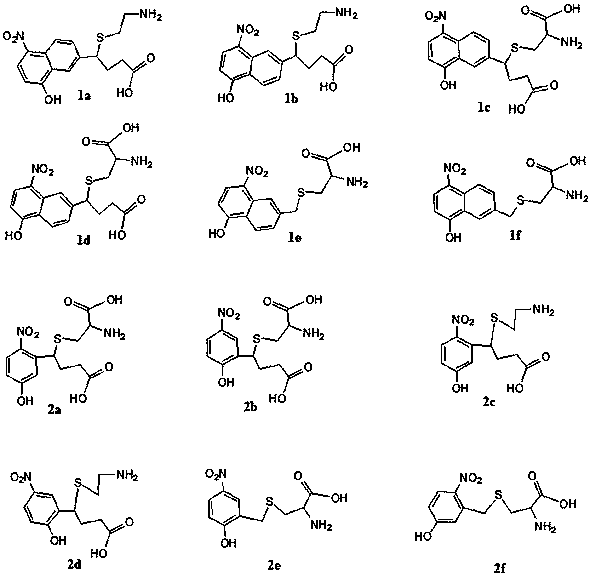
Amino acid having p-nitrophenol chromogenic group, and preparation method and applications thereof
A technology of p-nitrophenol and p-nitrophenol, which is applied in the preparation of sulfide, material analysis by observing the influence on chemical indicators, and analysis by making materials undergo chemical reactions, can solve the problem of immobilized chain affinity. Problems such as unsuitable, time-consuming, poor stability of probes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 5g of 2-hydroxy-5-nitrobenzyl alcohol into a 250ml three-necked flask, add 100 10% sulfuric acid and 10 g KBr, and react at 60°C for 6 hours; vacuum dry it with a rotary evaporator, and collect 2-hydroxy-5-nitrobenzyl alcohol Benzyl bromide, purified by silica gel column.
[0031] Add 2 g of the obtained product into a 250 ml three-necked flask, add 100 ml of dimethylformamide DMF, dissolve with magnetic stirring, then add 3 g of N-Boc cysteine, and reflux the reaction in the three-necked flask for 6 hours, and monitor to The chromophore has reacted. The crude product was vacuum-dried by a rotary evaporator, purified by silica gel column chromatography; deprotected by Boc with 1.0 M HCl and 10% trifluoroacetic acid to obtain the crude product of the candidate aminated small molecule probe; recrystallized twice with acetone to obtain Small molecule aminated chromogenic probe.
Embodiment 2
[0033] Add 1 g of biotin into a 50 ml flask, add 10 ml of DMF, stir magnetically at 80 degrees and heat to dissolve. Cool to room temperature, then add 1.1 times the mass of NHS and 2.0 times the mass of DCC, stir to dissolve, and react at room temperature overnight; filter, add 5 times the amount of ether to the supernatant to precipitate, and obtain the crude biotin active ester; reconstitute with 80ml of isopropanol Crystallized twice to obtain biotin active ester.
[0034] Add 1.0 g of the small-molecule amination probe obtained in Example 1 into a 250 ml three-necked flask, add 100 ml of DMF, and dissolve with magnetic stirring at 50 degrees. Add 5 ml of 0.5 g biotin active ester DMF solution, and stir for 1 hour at room temperature with magnetic stirring. Concentrate to the minimum volume with a rotary evaporator, add 5 times the volume of ether for precipitation; dissolve with DMF, and wash with ether for three cycles to obtain small molecule biotinylated chromogenic p...
Embodiment 3
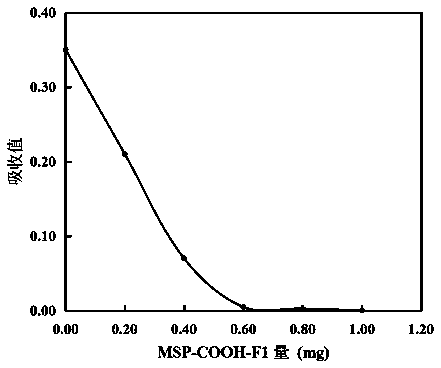
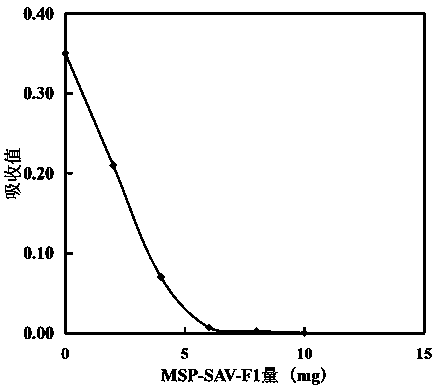
[0035]Example 3 Determination of activated carboxyl groups in carboxyl magnetic beads MSP-COOH-F1 by small molecule chromogenic probes
[0036] (1) A small molecule aminated chromogenic probe, prepared according to Example 1;
[0037] (2) When pH~8.0, the extinction coefficient of this chromogenic probe is about 16.8 (mM) at 405 nm -1 cm -1 ;
[0038] (3) Dissolve EDC and NHS with pH 6.0 25mM MES solution at a concentration of 25mg / ml, activate carboxyl magnetic beads MSP-COOH-F1 for 30min at a constant temperature of 27 degrees, and wash the activated magnetic beads with ice pH 6.0 25mM MES buffer 2 Repeat, and finally suspended in this pH 6.0 25mM MES buffer; store at 4 degrees and measure within 20 min;
[0039] (4) Dissolve and dilute the small molecule aminated chromogenic probe into pH 6.0 25mM MES buffer, the final concentration is 20.0μM, and its initial absorption is 0.350; prepare 15 ml chromogenic probe solution for each batch of dilution;
[0040] (5) Test tube...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Extinction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com