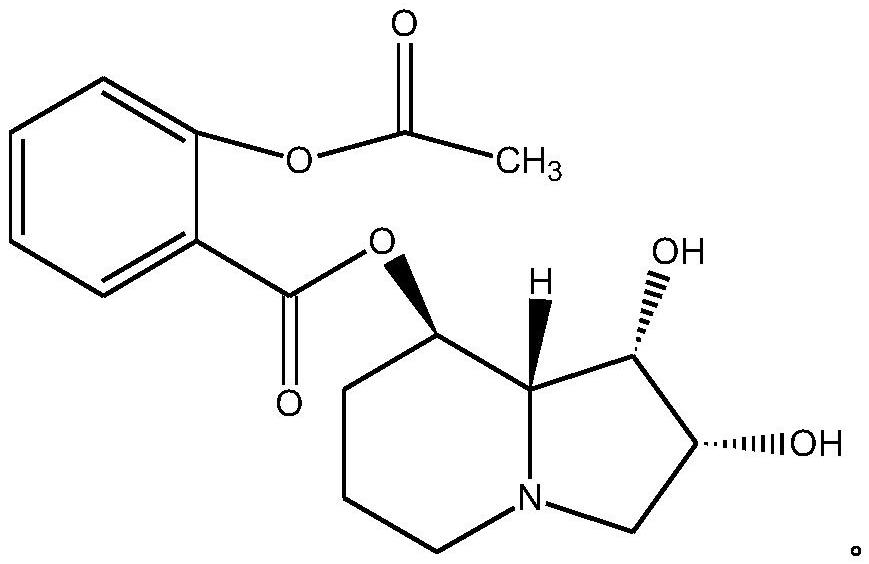
A kind of swainsonine derivative and its preparation method and application
A technology of swainsonine and its derivatives, which is applied in organic chemical methods, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of unstable properties of swainsonine and restrictions on popularization and application, and achieve easy operation , strong antiviral activity, simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] Preferred embodiments of the present invention are described below, and it should be understood that the preferred embodiments described here are only used to illustrate and explain the present invention, and are not intended to limit the present invention.
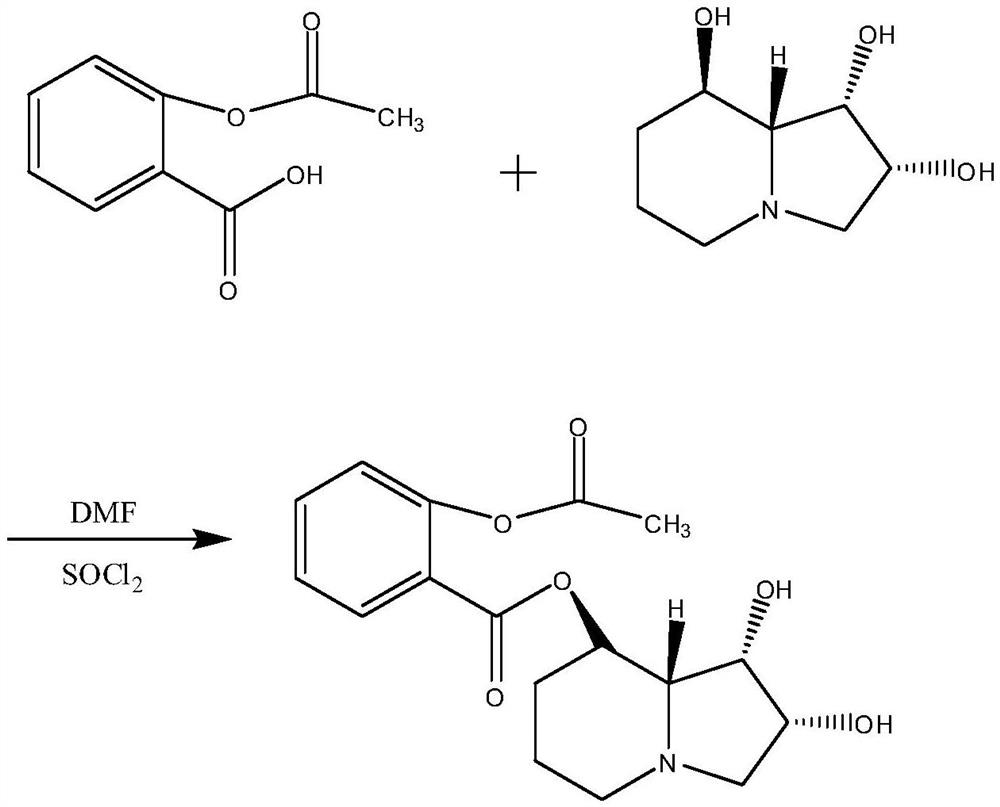
[0017] The preparation of embodiment 1 swainsonine derivatives
[0018] 1.1 Experimental part
[0019] 1.1.1 Main instruments and reagents
[0020] Avance 400 nuclear magnetic resonance spectrometer, Tensor 27 infrared spectrometer (Bruker, Germany); DSQ II mass spectrometer (Thermo, USA); X-6 precision microscopic melting point tester (Beijing Allen Electromechanical Technology Research Institute) .
[0021] Aspirin, DMF, SOCl 2 (analytical grade, Sinopharm Group Chemical Reagent Co., Ltd.); swainsonine (≥99%, industrial product, Shanghai Yiyan Biotechnology Co., Ltd.); ethyl acetate, petroleum ether (60-90°C) and other reagents are commercially available Sales of analytically pure; ordinary column chromatogra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com