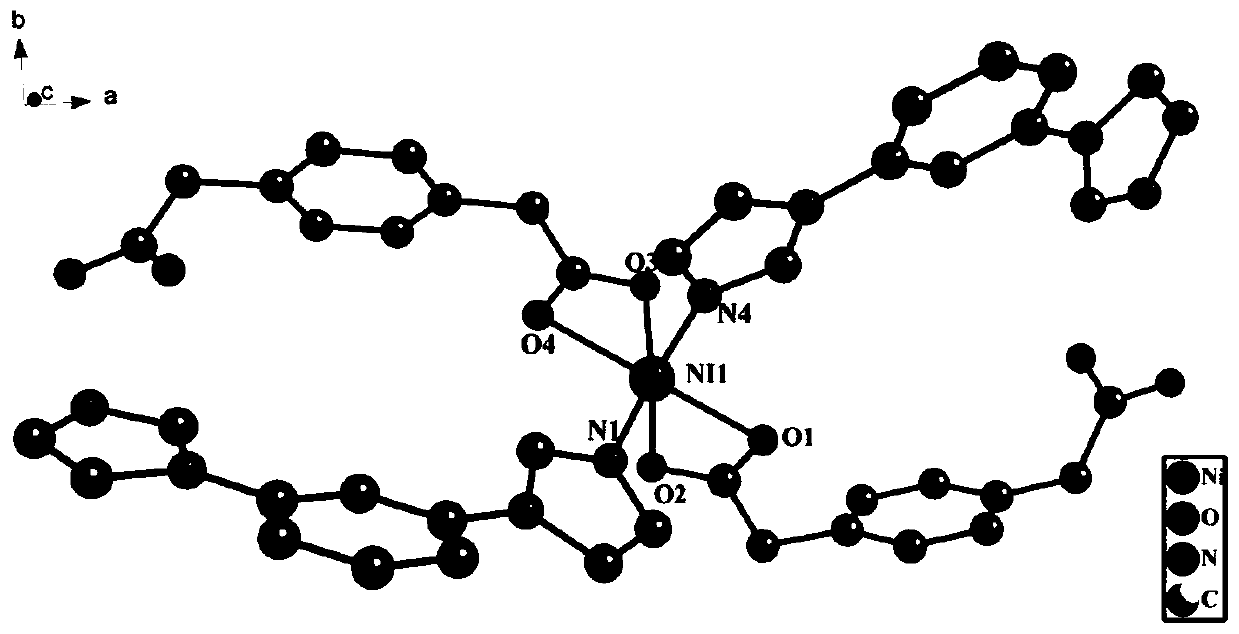
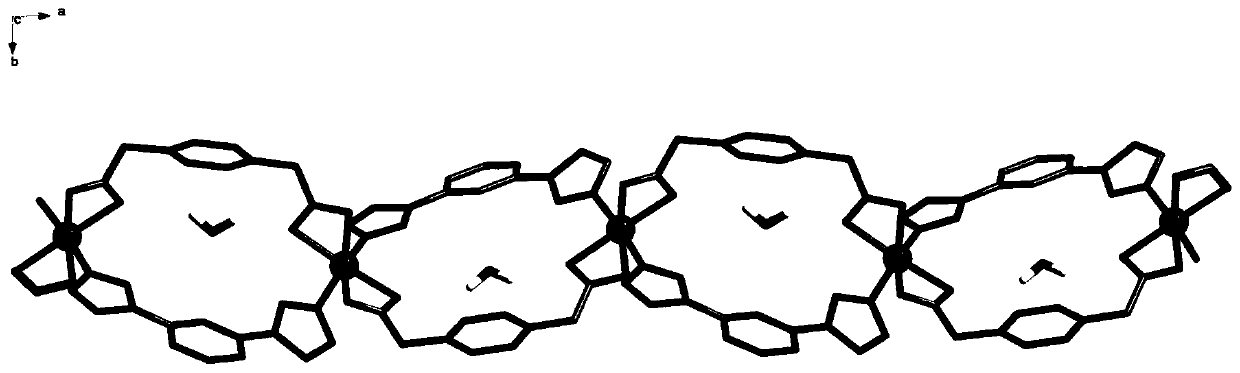
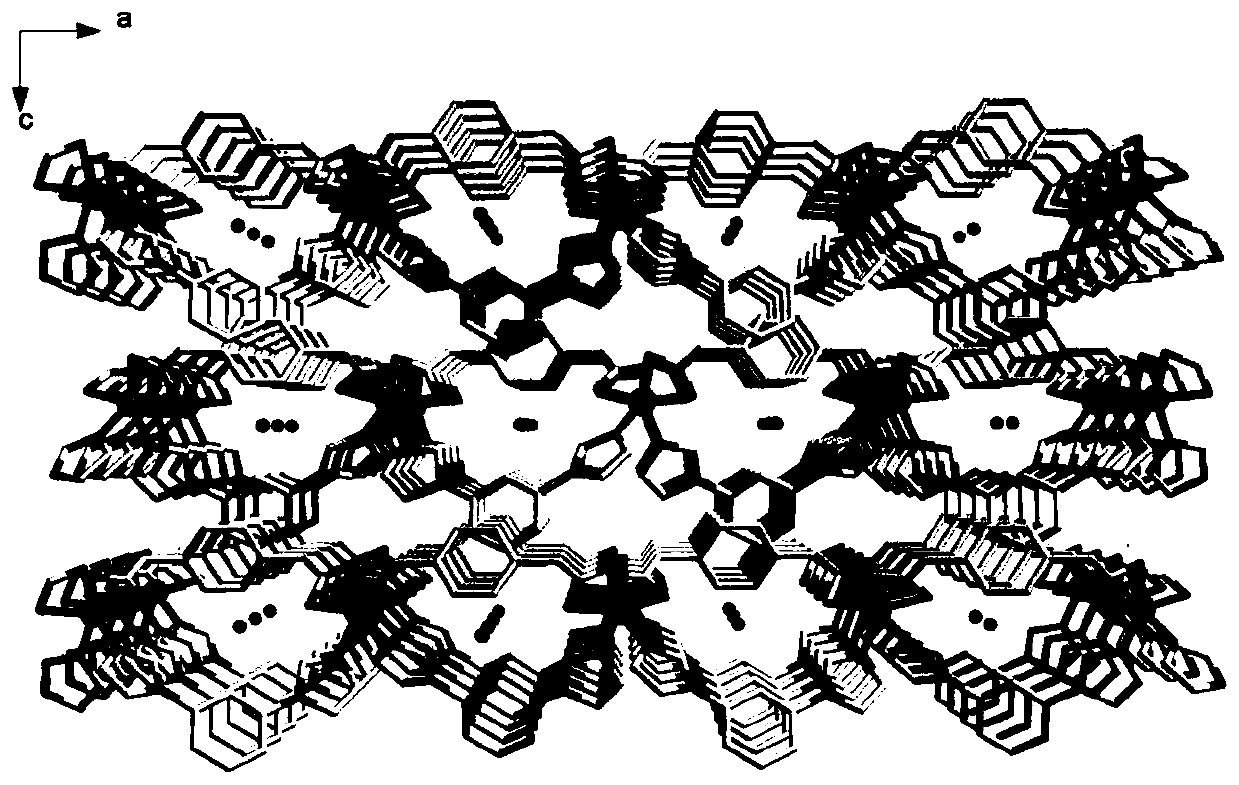
A kind of nickel metal coordination polymer and preparation method thereof
A coordination polymer and metal coordination technology, applied in the direction of hybrid capacitor electrodes, etc., can solve the problems of complex preparation process and low reproducibility, and achieve the effect of simple operation, good reproducibility and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] A kind of preparation method of nickel metal coordination polymer of the present invention, concrete preparation process comprises the following steps:
[0028] Step 1. Weigh an appropriate amount of nickel acetate, terephthalic acid, 1,3-bis(1-imidazolyl)benzene and an appropriate amount of deionized water in a 25mL beaker, stir evenly, and add an appropriate amount of KOH obtains mixed solution I;
[0029] In step 1, the molar concentration ratio of nickel acetate, terephthalic acid, 1,3-bis(1-imidazolyl)benzene and KOH is 1:1:1:1.
[0030] Step 2. Put the mixed solution I in a 25mL reaction kettle, and in the electrothermal constant temperature blast drying oven, first heat it quickly to a certain temperature, and react at a constant temperature for a period of time, then cool down to room temperature, take out the reaction kettle to obtain the mixed solution II, The mixed solution II was filtered, washed and dried to obtain emerald green bulk crystals, ie nickel me...
Embodiment 1
[0037] Weigh 0.1mmol of nickel acetate tetrahydrate, 0.1mmol of terephthalic acid, and 0.1mmol of 1,3-bis(1-imidazolyl)benzene, add 12mL of distilled water to a 25mL beaker, and add 0.1mmol of KOH to obtain mixed solution I, put mixed solution I into a 25mL stainless steel high-pressure reactor lined with polytetrafluoroethylene; Raise the temperature from 20°C to 200°C, perform a rapid heating reaction, react at a constant temperature of 200°C for 72 hours, then cool down to room temperature at a rate of 10°C / h, filter, wash and dry the reaction solution to obtain an emerald green massive crystal complex. That is, {[Ni(ppda)(mbib)]·H 2 O} n Complexes.
Embodiment 2
[0039]Weigh 0.1mmol of nickel acetate tetrahydrate, 0.1mmol of terephthalic acid, and 0.1mmol of 1,3-bis(1-imidazolyl)benzene, add 12mL of distilled water to a 25mL beaker, and add 0.1mmol of KOH to obtain mixed solution I, put mixed solution I into a 25mL stainless steel high-pressure reactor lined with polytetrafluoroethylene; Raise the temperature from 20°C to 180°C, perform a rapid heating reaction, react at a constant temperature of 180°C for 48 hours, then cool down to room temperature at a rate of 12°C / h, filter, wash and dry the reaction solution to obtain an emerald green massive crystal complex , namely {[Ni(ppda)(mbib)]·H 2 O} n Complexes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com