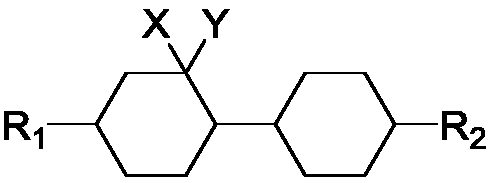
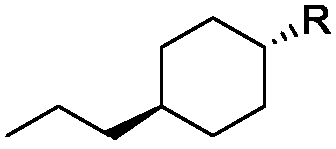
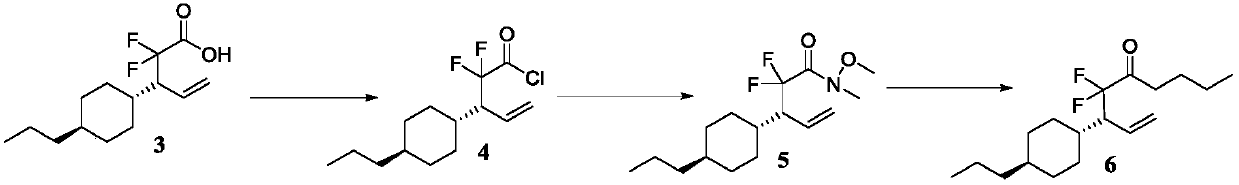
Geminal difluoro bicyclohexyl liquid crystal material and synthesis method of intermediate of material
A technology of bicyclohexane and liquid crystal materials, applied in the direction of liquid crystal materials, organic chemical methods, chemical instruments and methods, etc., can solve the problems of cumbersome preparation process and high cost of raw materials, and achieve simple process, low cost of synthesis process and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]
[0054] Take a 2L three-necked round-bottom flask, a water separator, and a reflux condenser, and add magnetons and difluorochloroacetic acid (64g, 493mmol, 1.3eq.). Add n-hexane (1L), add compound 1 (69g, 379mmol, 1eq.) dropwise at room temperature, raise the temperature to 90°C and reflux to separate water. After 18 h, the reaction was completed, cooled to room temperature, and water (500 mL) was added using 1N NaOH (aq .) Adjust Ph=7. Separate the layers, extract with EA (ethyl acetate), wash the organic phase with water and saturated brine successively, and dry over anhydrous sodium sulfate. Spin-dried to obtain compound 2 (colorless liquid, 89 g, yield 80%). 1 H NMR (500MHz, Chloroform-d) δ5.90-5.80 (m, 1H), 5.55 (dtd, J = 15.3, 6.8, 1.4Hz, 1H), 4.76 (d, J = 6.7Hz, 2H), 1.95 ( dddd, J=15.1, 11.2, 6.8, 3.5Hz, 1H), 1.81-1.71(m, 4H), 1.31(h, J=7.0Hz, 2H), 1.20-1.05(m, 5H), 0.95-0.83( m,5H). 19 F NMR (376MHz, Chlorof orm-d): δ–63.85(s, 1F). 13C NMR (101MHz, C...
Embodiment 2
[0057]
[0058] Take a 2L three-neck round bottom flask, reflux the condenser, add zinc powder (59g, 909mmol, 3eq.) and magneton. Under nitrogen protection, anhydrous acetonitrile (1 L) and TMSCl (66 g, 606 mmol, 2 eq.) were added. Compound 2 (89g, 303mmol, 1eq.) was added dropwise at room temperature, and the temperature was raised to reflux at 120°C. After 72h, the reaction was completed, and the insoluble matter was removed by filtration. Spin dry, add 6N HCl (aq.) (600mL) and stir for 1h, extract with EA; spin dry, add 2N NaOH (aq.) to adjust pH=14, Et 2 O extraction; 6N HCl (aq.) was used again to adjust pH=1, and EA extracted. The organic phase was washed successively with water and saturated brine, and dried over anhydrous sodium sulfate. Spin-dried to obtain compound 3 (black liquid, 51 g, yield 65%). 1 H NMR (400MHz, Chloroform-d) δ8.71 (s, 1H), 5.69 (dt, J = 17.0, 10.1Hz, 1H), 5.37-5.12 (m, 2H), 2.64 (dddd, J = 22.1, 12.2 ,10.0,4.2Hz,1H),1.86(dt,J=13.0,3.1Hz,...
Embodiment 3
[0061]
[0062] Take a 500mL round-bottom flask, add magneton and compound 3 (12.9g, 49.6mmol, 1eq.). Under nitrogen protection, DCM (248mL) and DMF (1.1g, 14.9mmol, 0.3eq.) were added, oxalyl chloride (9.4g, 74.4mmol, 1.5eq.) was added dropwise at 0°C, and stirred at room temperature for 13h. After the reaction is completed, spin dry and vacuumize.
[0063] Nitromethylhydroxylamine hydrochloride (7.3g, 74.4mmol, 1.5eq.) and magnetons were added to the above system. Under nitrogen protection, DCM (300 mL) was added, triethylamine (15.0 g, 148.8 mmol, 3 eq.) was added dropwise at 0° C., stirred at room temperature for 2 h, and the reaction was completed. Water (150 mL) was added to quench the reaction. Extract with DCM (dichloromethane), wash the organic phase with water and saturated brine successively, and dry over anhydrous sodium sulfate. Column chromatography (PE:EA=50:1) gave compound 5 (pale yellow liquid, 13.8 g, 92%). 1 H NMR (400MHz, Chloroform-d) δ5.71 (dt, J ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com