Method for preparing (R)-(4-chlorophenyl)oxirane from recombinant escherichia coli
A technology for recombining Escherichia coli and Escherichia coli, applied in the biological field, can solve the problems of harsh reaction conditions, weak stereoselectivity, toxicity and the like, and achieve the effect of strong enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
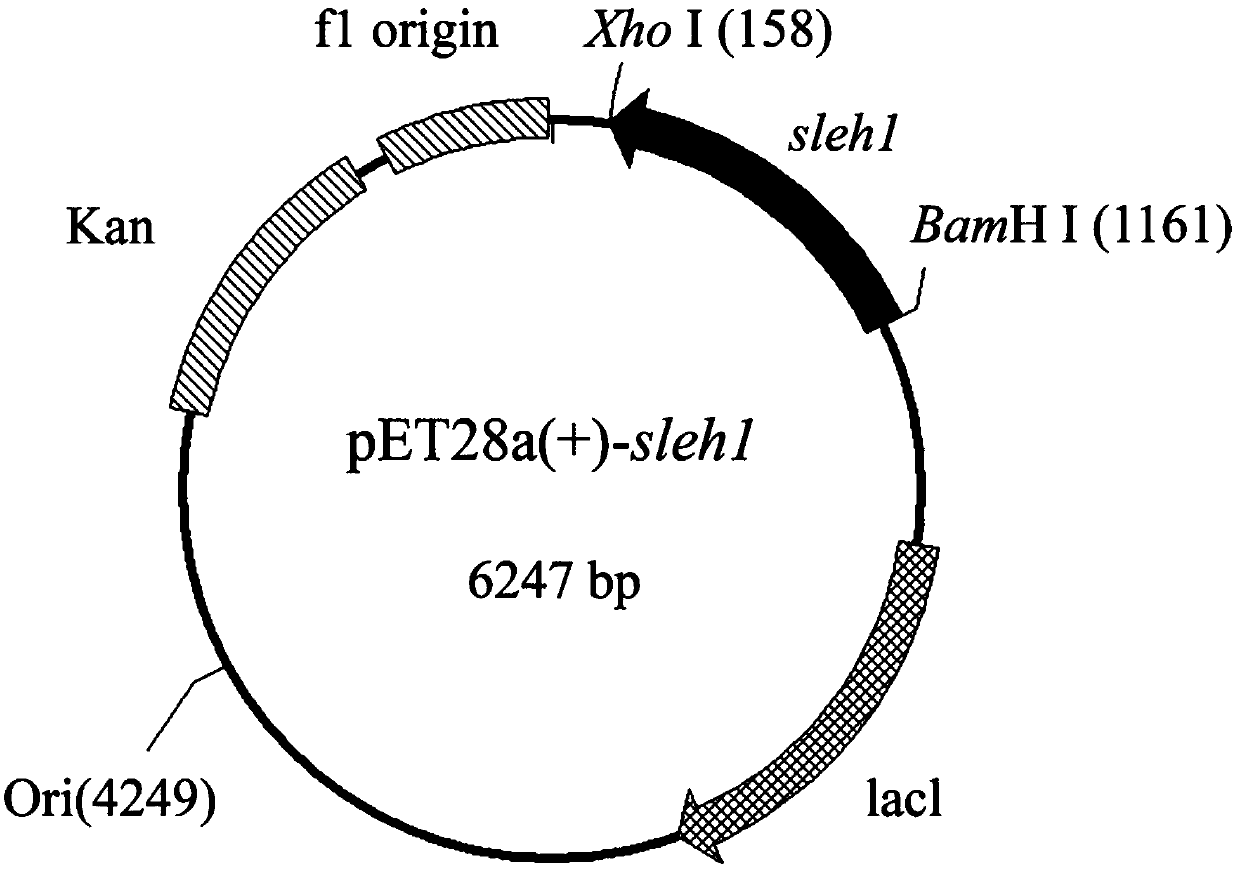
[0029] The cloning of embodiment 1 epoxide hydrolase gene and the construction of expression plasmid
[0030]The total RNA of tomato was extracted (using TRIZOL kit, Invitrogen Company), and the operation was performed according to the instructions of the kit. The reverse transcription process refers to the instructions of the RT-PCR kit (PCR PrimeScriptTM RT reagent Kit) from Takara Company, and then the cDNA obtained by reverse transcription is used as a template, and the upstream and downstream primers (the BamHI and XhoI restriction endonuclease enzymes are introduced into the primers) Cutting sites (synthesized by Shanghai Sangon)) sequences were GGATCCATGGAGAAGATAGAGCACAAGAT, CTCGAGGAACTTTTGAATGAAGTCATAGATGTG, and the full-length sleh1 gene was amplified by conventional PCR method. Extend at ℃ for 60s, cycle 30 times, and extend at 72℃ for 10min. The PCR product was detected by 1.0% agarose nucleic acid gel electrophoresis and after purification, the target fragment was...
Embodiment 2
[0031] Example 2 Effect of Temperature on Induced Expression of Recombinant Strain
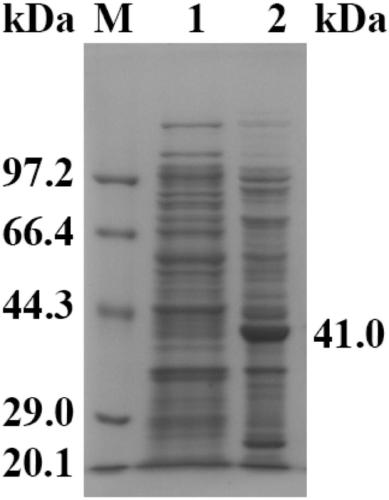
[0032] Inoculate the genetically engineered bacteria E.coli / sleh1 in Example 1 in LB liquid medium containing 0.1g / L kanamycin, culture at 37°C and shake at 220rpm for 12h, then inoculate the culture solution at 1% Inject a large amount into LB medium, culture at 220 rpm at 37°C for 2.5 hours, then add IPTG at a concentration of 0.2 mM, culture at 220 rpm at 16-30°C for 8 hours, and collect the bacteria by centrifugation.
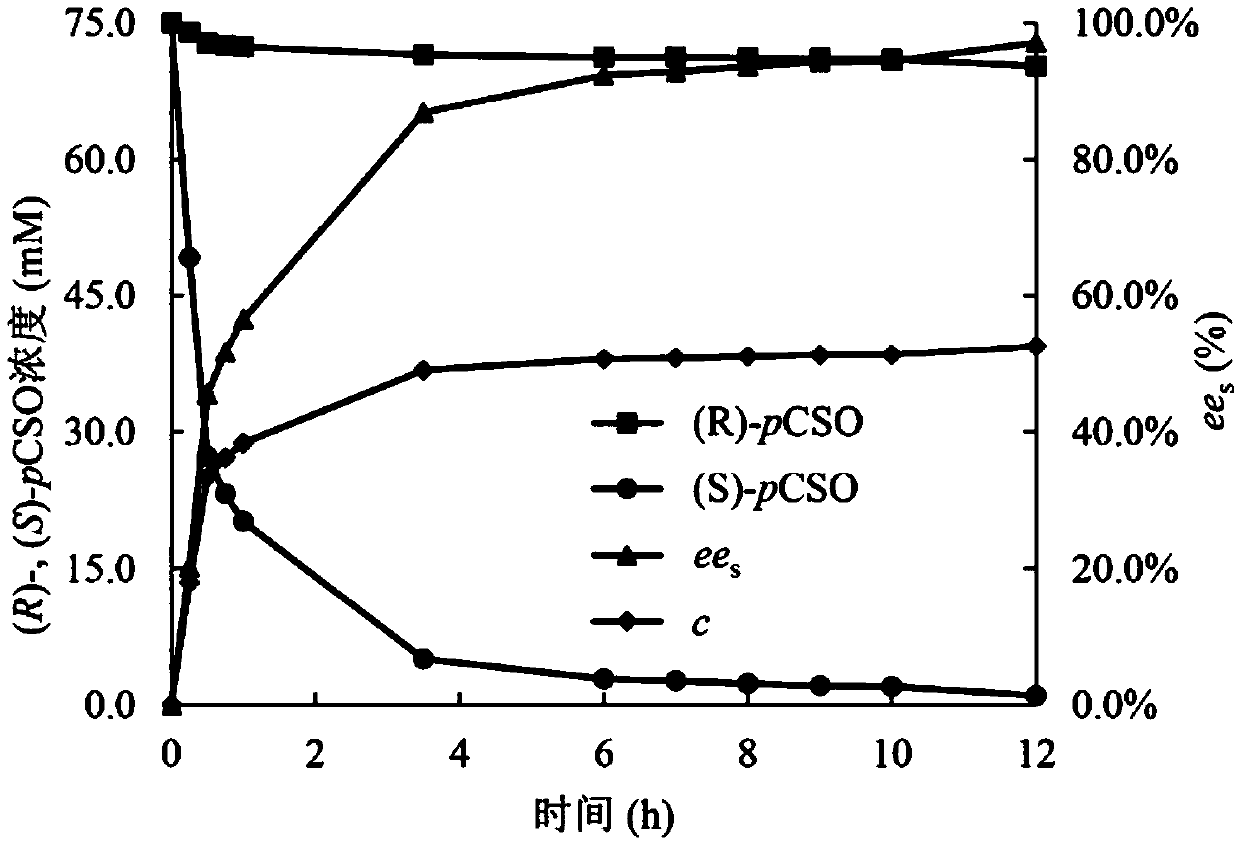
[0033] The obtained cells were treated with NaH according to the concentration of 150mg / mL wet cells 2 PO 4 -Na 2 HPO 4 (100mM, pH 7.5) buffer for resuspension. The bacterial suspension was directly used as a catalyst, and rac-pCSO was used as a substrate to compare the catalytic activity of living cells at different induction temperatures. The specific operation is: in a 10mL Ep plastic tube, add 3.3mL of bacterial suspension, 0.5mL of rac-pCSO methanol solution with ...
Embodiment 3
[0036] Embodiment 3 induction time influences the induced expression of recombinant strain
[0037] Inoculate the genetically engineered bacteria E.coli / sleh1 in Example 1 in LB liquid medium containing 0.1g / L kanamycin, culture at 37°C and shake at 220rpm for 12h, then inoculate the culture solution at 1% The amount was added to LB medium, shaken and cultured at 220 rpm at 37°C for 2.5h, then added with 0.2mM IPTG, shaken at 220rpm at 25°C for 4-16h, and collected by centrifugation.
[0038] The comparison method is the same as above, with the relative enzyme activity at the induction temperature of 8 hours as 100%, the catalytic activity of the living cells under different induction times is compared, and the results are shown in Table 2. The relative enzymatic activity of the epoxide hydrolase in the recombinant Escherichia coli cells after induction for 8-16 hours reaches over 80.1%.
[0039] Table 2
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com