Preparation method and application of CAR-T cell based on base editing target CD133
A technology of CD133-CAR and cd133-scfv, which is applied in the field of immunology and immunotherapy, can solve the problems of complex immune drug preparation process, high cost, and potential safety hazards, and achieve low off-target rate, high integration efficiency, and enhanced load capacity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Preparation of PBMC cells.
[0062] Take 15 mL of human peripheral blood, treat with anticoagulant (EATA or heparin), centrifuge at 350×g for 5 minutes at room temperature for 15 minutes, and aspirate the plasma for use.
[0063] Add 1 volume of PBS buffer to the blood cells to dilute and mix well, take a 15mL centrifuge tube, add 5 mL of lymphocyte separation medium Ficoll, and slowly add 5 mL of diluted blood cells to the upper layer. This step must be slow to prevent Ficoll from mixing with blood cells.
[0064] Slowly rise and fall, centrifuge at room temperature 450×g for 25 minutes, the blood cells are divided into platelet layer, white blood cell layer (buffy coat), polysucrose (Ficoll) layer and red blood cell layer from top to bottom, suck platelet layer (2mL), and white blood cell layer (Buffy coat, about 3 mL) was transferred to a new 15 mL centrifuge tube.
[0065] Add 10 mL of PBS buffer, centrifuge at 300*g for 10 minutes, discard the supernata...
Embodiment 2
[0066] Example 2: Codon optimization.
[0067] (1) Prepare the CD133 chimeric antigen receptor (CAR), namely CD133-CAR, and send the scFv fragment to the company for codon optimization to make it easier to express in human cells. The codon-optimized sequence is SEQ ID NO .1 the nucleotide sequence shown.
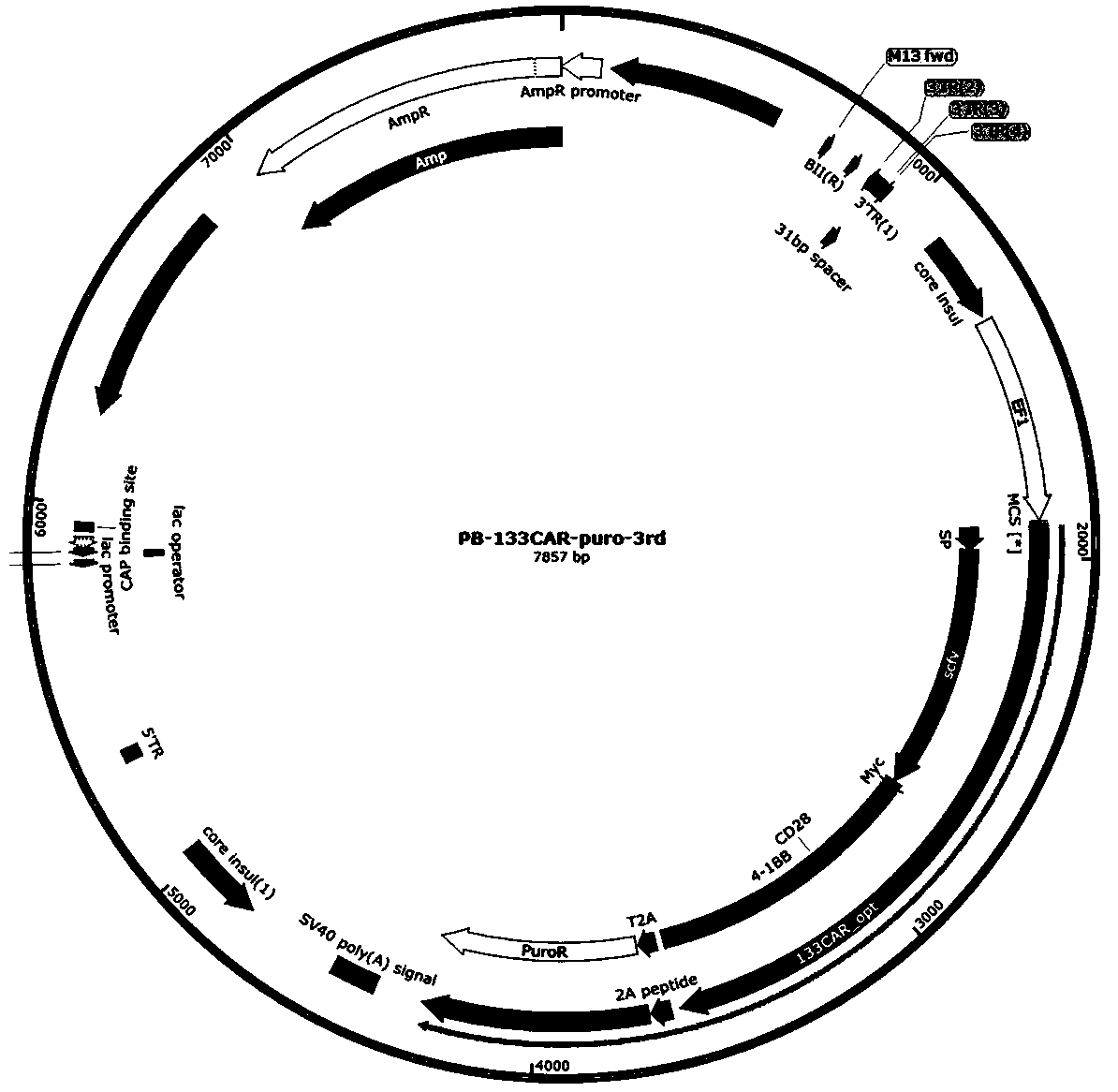
[0068] (2) Synthesize the target fragment, use In-Fusion technology to fuse the synthetic fragment to the PB-133CAR-puro vector, and make the PB-CD133-CAR-BBZ-puro plasmid (such as figure 2 shown), the DNA sequence is the nucleotide sequence shown in SEQ ID NO.2.
[0069] Synthetic primers Primer-F: CGGCGCCTACTCTAGATGCTGCTGCTGGTGACCCTCTCTGC and Primer-R: GGGGACGAACAGATCAGATCCGGCTGCAGCGGC. The synthesized CD133 optimized sequence was amplified by PCR, and the CD133 PCR product was recovered.
[0070] The original PB-CD19 CAR-puro vector was double-digested with Xba I and Bgl II restriction endonucleases, followed by the steps of the TaKaRaIn-Fusion kit, the templates were...
Embodiment 3
[0072] Example 3: Using the BE-Plus system to prepare CAR-T cells targeting CD133 and knocking out the PD-1 gene
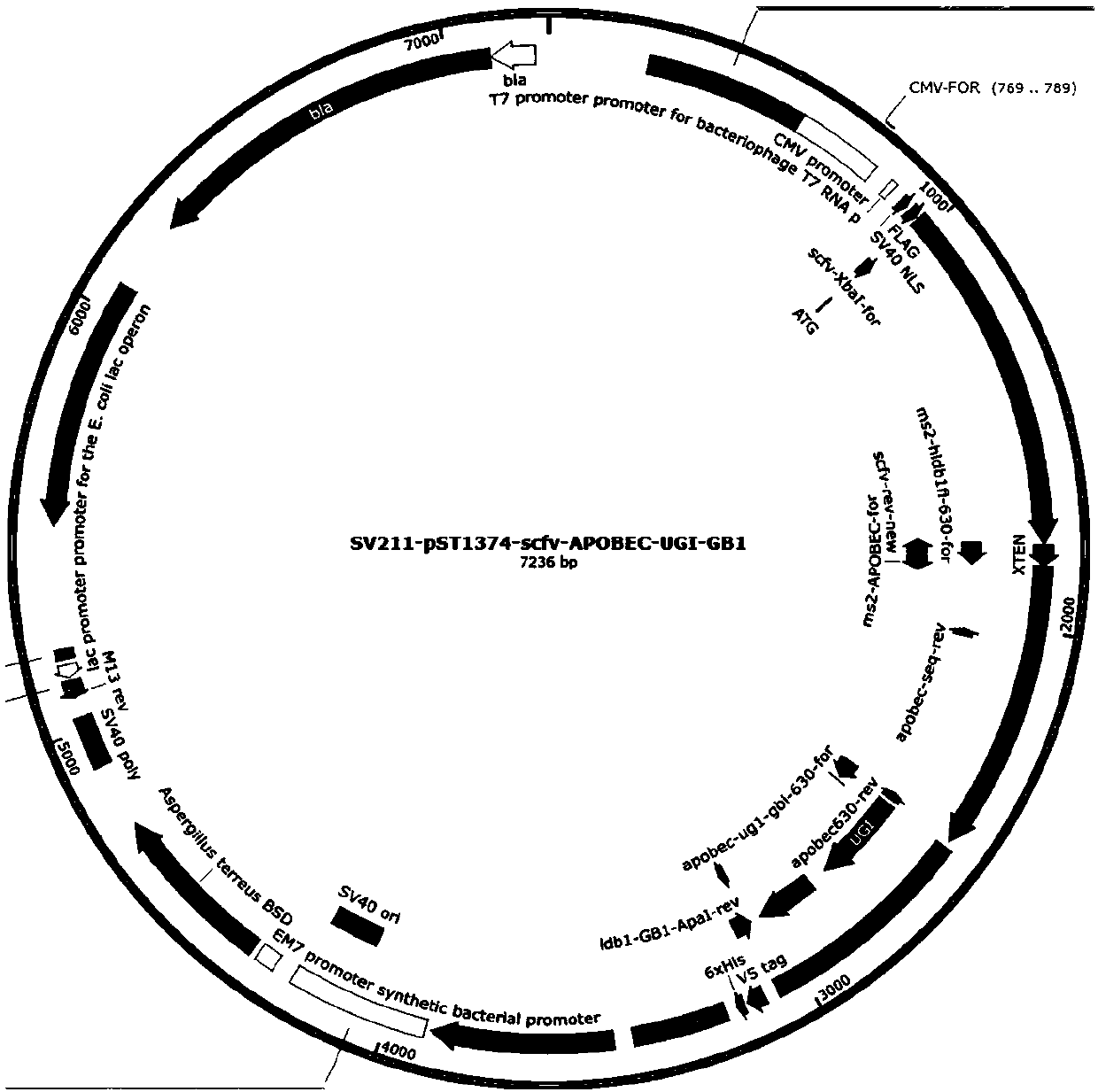
[0073]Based on the efficiency of BE-Plus-mediated single base editing, design a gene knockout strategy: introduce stop codons through CT mutations, such as mutating CAA, CAG, and CGA into stop codons TAA, TAG, and TGA, to stop coding Gene translation, thereby achieving gene knockout. Or reverse CT mutation, the CCA codon is mutated to TCA, CTA, and its reverse complementary codon TGG is mutated to TGA, TAG, BE-Plus dual plasmid system (pST1374-scfv-APOBEC-UGI-GB1 (such as image 3 shown), pST1374-N-NLS-GCN4-D10A (as shown Figure 4 As shown)), 10 GCN4s were connected to the N-terminal of D10A, and one scFv was connected to the N-terminal of APOBEC. In this way, GCN4-D10A can recruit 10 scFv-APOBECs, increasing the probability of mutation. The work window is expanded from 4-8 to 4-16, making the efficiency higher.
[0074] Select the 20bp-NGG target sequence (P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com