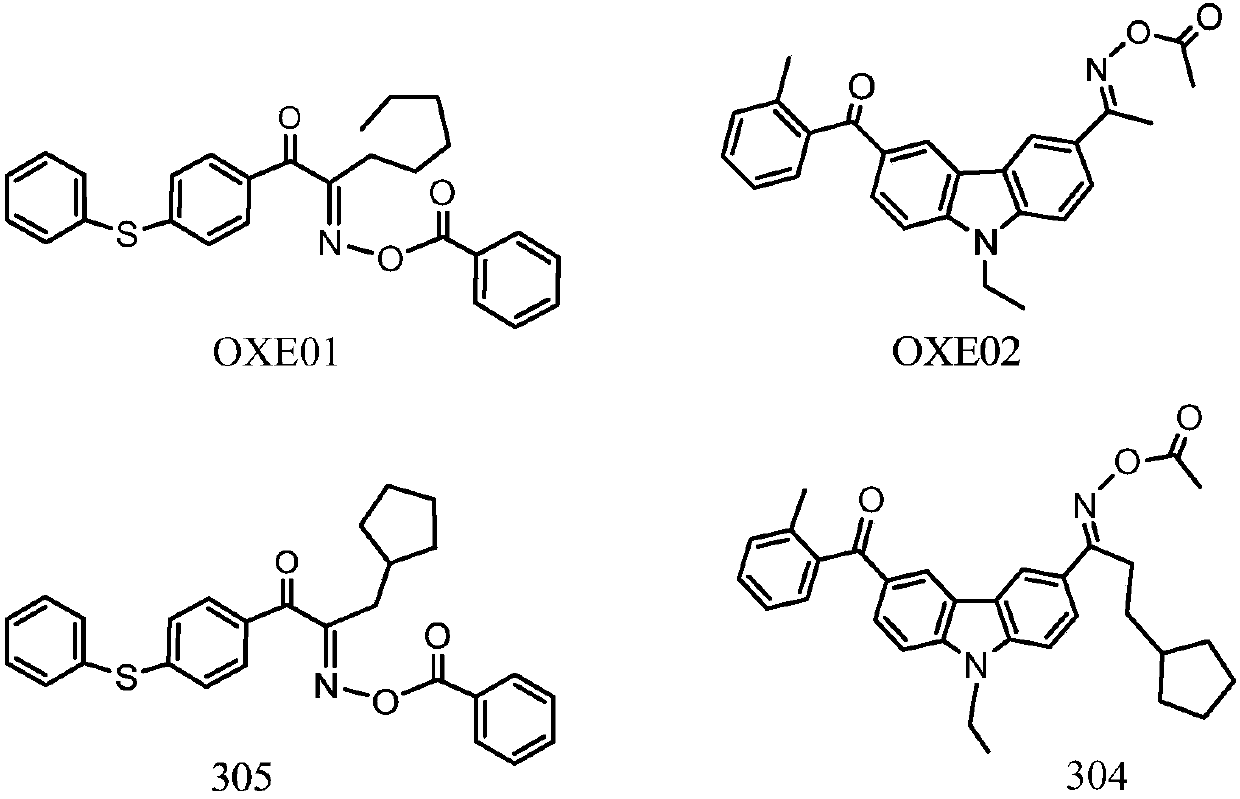
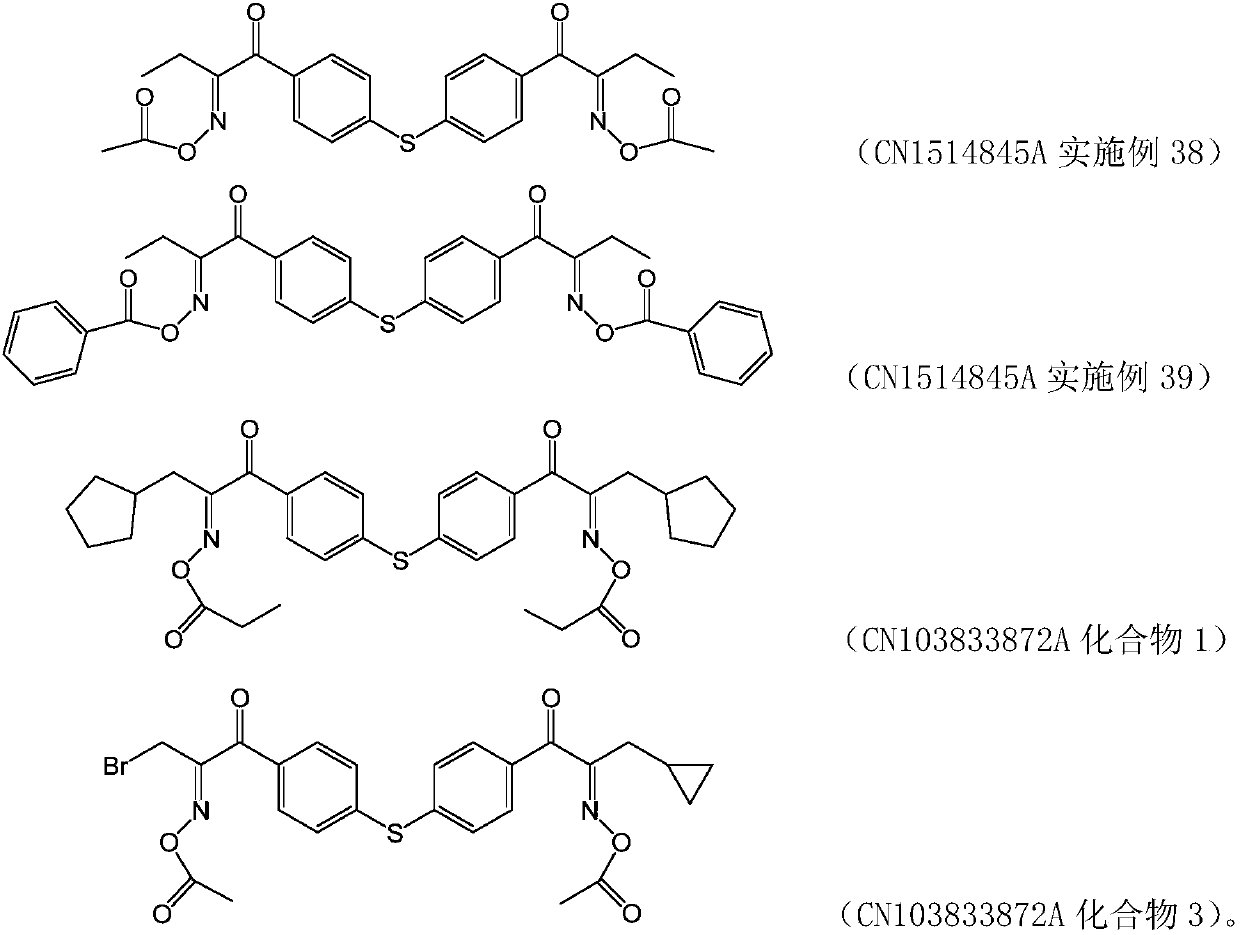
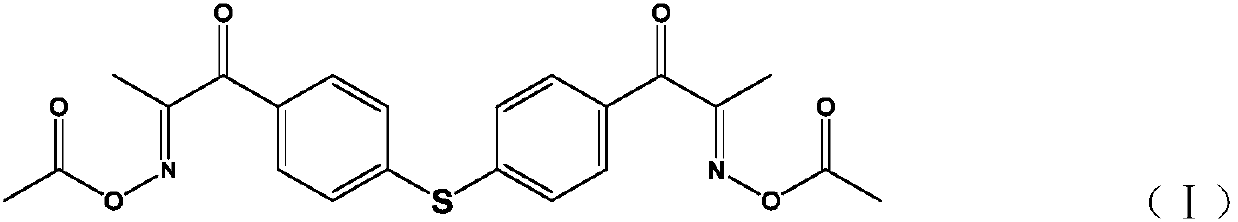
Diketoxime ester compound, preparation method and applications thereof
A manufacturing method and compound technology, which are applied in the field of diketoxime ester compounds, can solve the problems that the yellowing of carbazole oxime ester cannot meet the requirements of color difference of color photoresists, etc., and achieve excellent anti-oxygen polymerization inhibition, high photosensitivity, clear imaging effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 synthetic formula I compound
[0052] 1-1 Synthesis of 4,4'-dipropionyl diphenyl sulfide (compound of formula II)
[0053] Weigh 79.0g (0.424mol) of diphenyl sulfide and dissolve it in 600g of 1,2-dichloroethane, add 124.4g (0.933mol) of anhydrous aluminum trichloride, stir and cool down to 0°C; keep below 5°C , add propionyl chloride 82.4g (0.891mol) dropwise, after 2 hours of dropping, continue to keep stirring at 0°C for 2h; add the reaction liquid dropwise to 450ml of 10% dilute hydrochloric acid, control the temperature of the acid solution not higher than 30°C, and stir for 1h after adding , separate the lower organic phase; the organic phase is washed three times with 300ml of water successively; the organic phase is subjected to vacuum distillation to recover the solvent, and the residue is 126g; 240ml of n-hexane is added while it is hot, and the insulation makes the solution clear; natural cooling makes it crystallize, and the solution When the t...
Embodiment 2
[0058] Embodiment 2 Alkali-soluble resin preparation
[0059] Mix 180g of benzyl methacrylate, 60g of methacrylic acid, 60g of hydroxyethyl methacrylate, 15g of azobisisobutyronitrile, 6g of dodecanethiol and 1000ml of toluene and put them into a constant pressure dropping funnel; Put 1000ml of toluene into a three-necked flask, install stirring, a constant pressure dropping funnel and a thermometer, start stirring, and replace the gas in the flask with nitrogen; heat the flask to make the solvent temperature reach 80-85°C, keep it warm, and start adding the monomer mixed solution dropwise, about After 1 hour of dripping; continue to react for 6 hours; naturally cool down, stop stirring, and wait for the resin to settle, absorb the upper clear solution, filter the lower solvent-containing resin, and rinse the resin filter cake with 500ml of toluene; dry the filter cake under reduced pressure to obtain a white Powdered solid resin 250g; it is dissolved into 20% solution with PM...
Embodiment 3
[0060] Preparation and development of embodiment 3 photoresist
[0061] According to the weight ratio of formulations 3A, 3B, 3C, and 3D in Table 1, all components were prepared into photocurable compositions according to the ink preparation method, which were in fluid liquid state.
[0062] Each of the above-mentioned liquid compositions was coated on the glass surface by the wire rod method, and baked at 80° C. for 3 minutes to evaporate the solvent PMA, and the film thickness of the remaining film was measured to be 2 microns for later use.
[0063] First set of exposure tests
[0064] Place a 21-step gray gradient ruler on the film, filter the 2000W high-pressure mercury lamp light with a 365nm grating filter, and set the distance between the film and the grating at 10cm to make the exposure amount reach 200mJ / cm 2 .
[0065] Soak in a 1% sodium carbonate solution bath at 30°C for 1min, and record the maximum film retention order that can be displayed. The larger the numbe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com