A kind of cordyceps militaris fibrinolytic enzyme and its preparation method and application
A technology of fibrinolysis and Cordyceps militaris, which is applied in the field of bioengineering, can solve problems such as retention, and achieve the effects of improving production efficiency, good thrombolytic activity, and shortening fermentation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Screening of high-enzyme live Cordyceps militaris strains
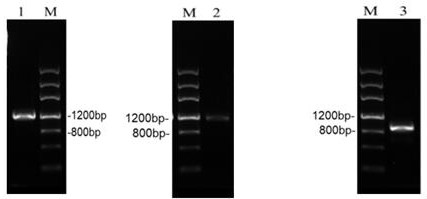
[0033] Activation of 10 strains of Cordyceps militaris was carried out using solid plate medium, and a piece was cut from the slope and placed on a solid improved PDA plate (add 2% peptone, 0.3% KH on the basis of ordinary PDA solid medium) 2 PO 4 , 0.15% MgSO 4 , 0.002% VB 1 ) center, cultured at 24°C in the dark for 10 days, the mycelium covered the plate and did not turn color. Liquid culture fermentation was carried out on the activated 10 strains of Cordyceps militaris.
[0034] The culture conditions are: put 50mL culture medium in a 250mL Erlenmeyer flask, add a piece of bacteria block with a side length of about 1cm, and culture in a shaking flask at 24°C and 150r / min in the dark. From the 5th day, sample 1 mL every day, centrifuge at 4°C and 10,000 r / min for 5 minutes, take the supernatant as a crude enzyme solution for enzyme activity detection, and monitor for 20 days. Finally, consider...
Embodiment 2
[0035] Example 2: Identification of Cordyceps militaris Fibrinolytic Enzyme
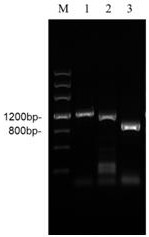
[0036] (1) N-terminal sequencing of plasmin: take the high-enzyme active Cordyceps militaris strain obtained in Example 1 as the research object, carry out liquid fermentation on it and separate and purify the crude enzyme solution, and electrophoresis the protein samples according to Table 2 The method runs the gel, and transfers the protein to the PVDF membrane after the end. Before transfer, soak gel and filter paper of appropriate size in CAPS buffer for 5-10min. PVDF membranes were soaked in methanol for a few seconds and then also placed in CAPS electroblotting buffer. During installation, filter paper, PVDF membrane, glue, and filter paper are followed from bottom to top to drive out air bubbles.
[0037] The semi-dry method was adopted, and the transfer conditions were: 25V constant voltage, 1A, 60min. After the transfer was completed, the PVDF membrane was stained with Coomassie Brilliant...
Embodiment 3
[0045] Example 3: Induced expression and separation and purification of recombinant Cordyceps militaris fibrinolytic enzyme
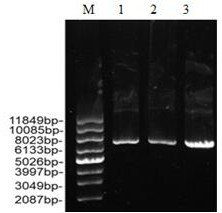
[0046] E. coli cells containing the recombinant plasmid were cultured in 250 mL shake flasks containing 50 mL of LB broth supplemented with 100 μg / mL ampicillin and grown at 37 °C and 200 rpm until reaching an optical density of 0.6 at 600 nm. The culture was then added to 50 μg / mL IPTG to induce enzyme production, and culture was continued at 20 °C for 20 h. After incubation, the culture was centrifuged at 10000 rpm for 10 min at 4°C. Cells were then resuspended in cell culture medium. Lysis buffer (50mM NaH 2 PO 4 , 300mM NaCl, 10mM Tris, 20mM imidazole, pH 8.0) and disrupted by sonication. The crude extract was then centrifuged at 10000 rpm for 20 min at 4°C. Since the C-terminus of the pEASY Blunt E2 vector has a 6×His tag, nickel column affinity chromatography was used to purify the expression product. After the bacteria liquid was fermented ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com