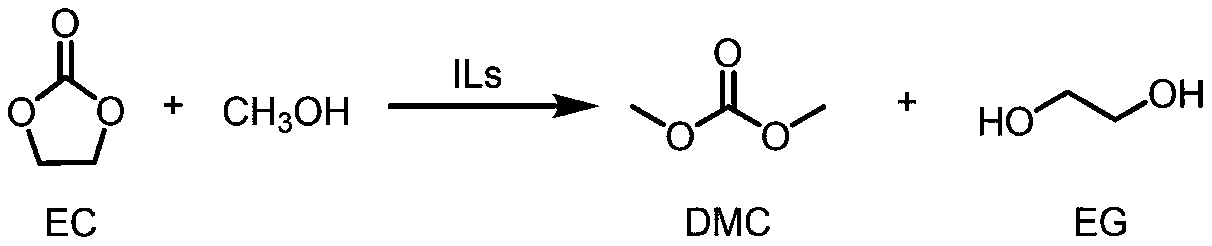
Method for synthesizing dimethyl carbonate under catalysis action of ionic liquid
A technology of dimethyl carbonate and ionic liquid, which is applied in the field of synthesis of dimethyl carbonate, can solve problems such as the complexity of the preparation process, and achieve high catalytic activity, simple synthesis operation, and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] To operate under normal pressure reflux conditions, in a 50mL round bottom flask, add ethylene carbonate (0.8806g, 10mmol), methanol (3.204g, 100mmol) and ionic liquid catalyst [DBUH][TFA] (3mol%, 0.3 mmol), under the condition of a stirring rate of 800r / min, the reaction temperature was raised to 60°C to start the reaction, and the reaction time was 7h. After the reaction was completed, the reaction system was analyzed by gas chromatography to obtain a conversion rate of ethylene carbonate of 45% and a yield of dimethyl carbonate of 20% (internal standard method, 1,3,5-trimethoxybenzene internal standard). The catalyst is separated from the reaction system by distillation under reduced pressure, and the catalyst is recovered.
Embodiment 2
[0026]To operate under normal pressure reflux conditions, the raw material ethylene carbonate (0.8806g, 10mmol), methanol (9.612g, 300mmol) and ionic liquid catalyst [DBUH][TFE] (0.1mol%, 0.01mmol) were sequentially added to a 50mL circle In the bottom flask, the reaction temperature was raised to the reflux temperature of the system at 70° C. under the condition of a stirring rate of 1000 r / min to start the reaction, and the reaction time was 12 hours. After the reaction was completed, the reaction system was analyzed by gas chromatography to obtain a conversion rate of ethylene carbonate of 85% and a yield of dimethyl carbonate of 83% (internal standard method, 1,3,5-trimethoxybenzene internal standard). The catalyst is separated by distillation under reduced pressure, and the catalyst is recovered.
Embodiment 3
[0028] Under normal pressure reflux condition, add raw material ethylene carbonate (0.8806g, 10mmol), methanol (1.602g, 50mmol) and ionic liquid catalyst [DBUH]Cl (2mol%, 0.2mmol) successively in 50mL round bottom flask , the reaction temperature was raised to 40° C. under the condition that the stirring rate was 600 r / min, and the reaction time was 2 h. After the reaction was completed, the reaction system was analyzed by gas chromatography to obtain a conversion rate of ethylene carbonate of 64% and a yield of dimethyl carbonate of 15% (internal standard method, 1,3,5-trimethoxybenzene internal standard). The catalyst is separated by distillation under reduced pressure, and the catalyst is recovered.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com