Preparation method of guineensine
A technology of piperine and compounds, applied in the field of drug synthesis, can solve the problems of unstable plant planting yield, high extraction cost, and the content of piperine in Guinea cannot meet the demand for drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
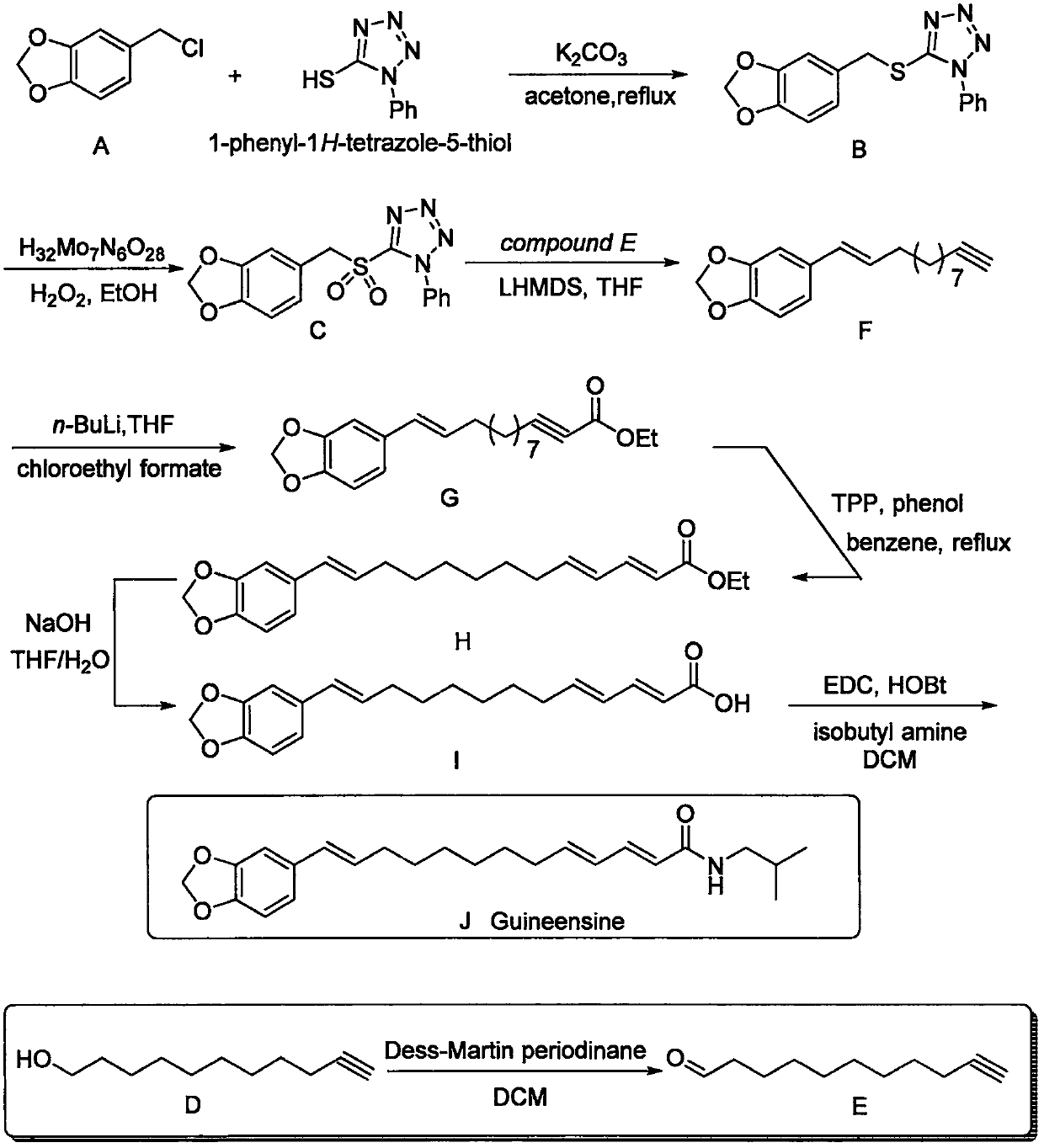
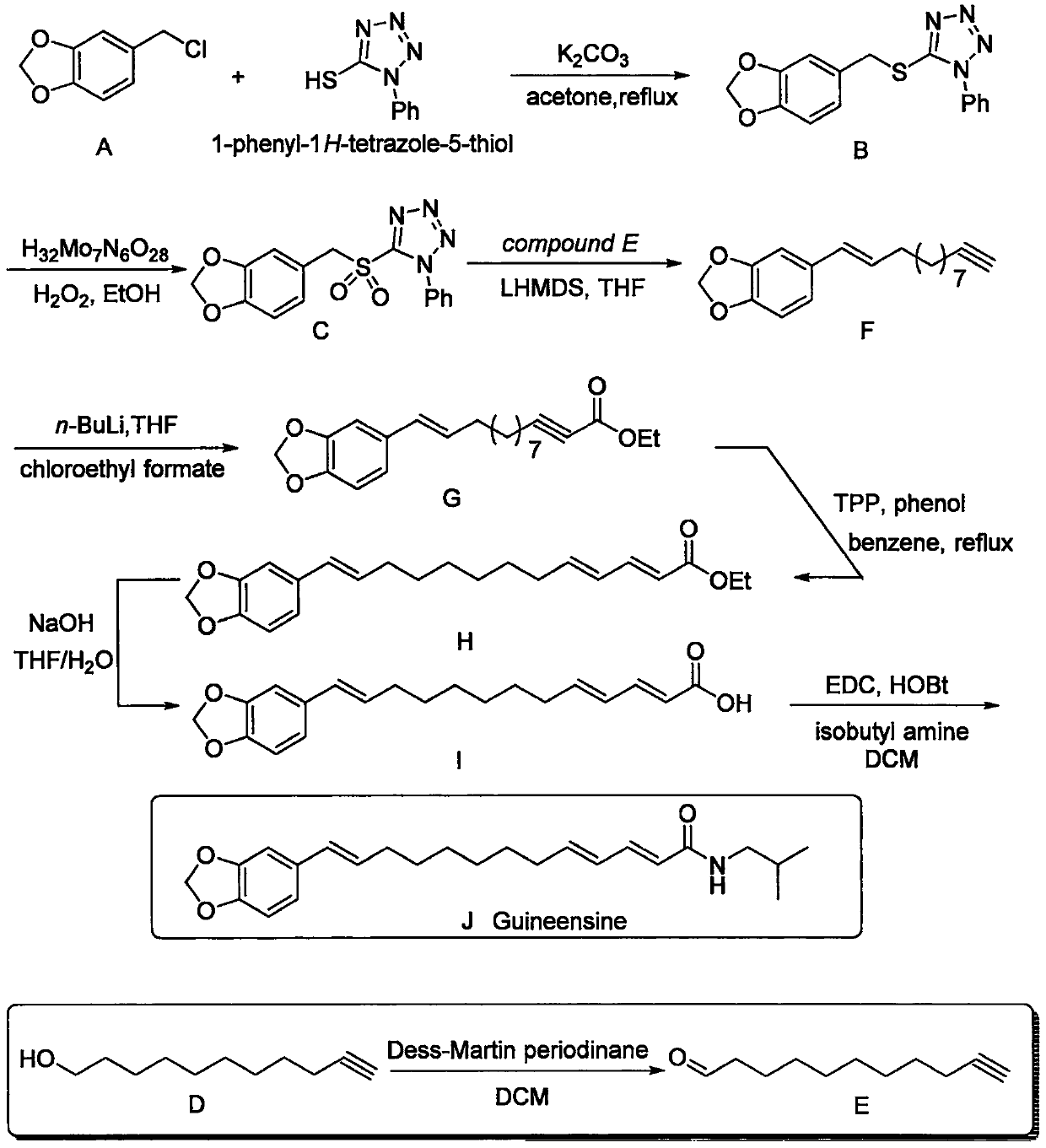
[0009] Such as figure 1 Shown, the preparation method of guinea piperine, comprises the steps:
[0010]
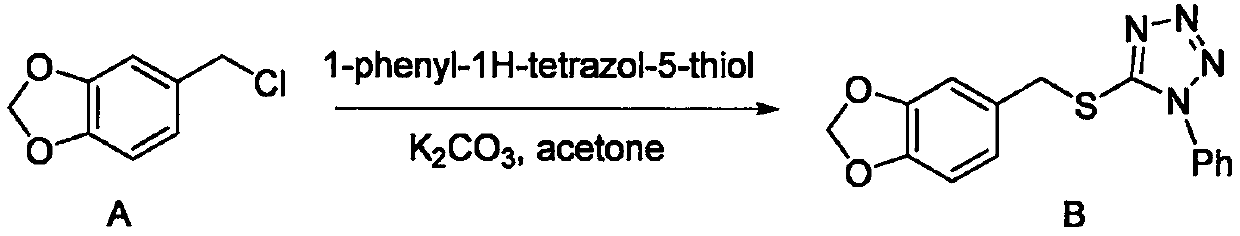
[0011] Compound A (1.0 equiv) was dissolved in acetone, 1-phenyl-1H-tetrazol-5-thiol (1.0 equiv) was added, followed by potassium carbonate (1.5 equiv). The reaction system was heated to reflux and reacted overnight. After the reaction, the reaction system was concentrated, acetone was removed, ethyl acetate was added to redissolve, filtered, potassium carbonate was removed, the organic phase was washed twice with water, the organic phase was dried, and concentrated to obtain the crude product B. The crude product B can be directly put into the next reaction.
[0012]
[0013] Compound B (1.0 equivalent) was dissolved in ethanol, ammonium heptamolybdatetetrahydrate (0.2 equivalent) was added, and the temperature of the reaction system dropped to 0°C. The same volume of hydrogen peroxide as ethanol was then added, and the reaction was stirred for 18 hours. After t...
Embodiment
[0027] The following is the specific example part, wherein, the proton nuclear magnetic resonance spectrum and the carbon nuclear magnetic resonance spectrum of the following compounds are all obtained by the method of nuclear magnetic resonance, and the instrument and test conditions used in the test are as follows: Brüker Advance 300 ( 1 H: 300MHz, 13 C: 75MHz), Brüker Advance 400 ( 1 H: 400MHz, 13 C: 100MHz), Brüker Advance 500 ( 1 H: 500MHz, 13 C: 125MHz), using TMS or the residual non-deuterated solvent in the deuterated solvent as internal standard. The preparation method of the Guinea piperine of the present embodiment is as follows:
[0028]
[0029] Compound A (50.8 g) was dissolved in acetone (700 mL), 1-phenyl-1H-tetrazol-5-thiol (53.9 g) was added, followed by potassium carbonate (62.7 g). The reaction system was heated to reflux and reacted overnight. After the reaction was finished, the reaction system was concentrated, acetone was removed, ethyl acetate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com