Bioactivity polypeptide and application thereof to preparation of alpha-glucosidases inhibitor
A bioactive peptide, glucosidase technology, applied in the fields of peptides, DNA/RNA fragments, recombinant DNA technology, etc., can solve problems such as side effects and metabolic disorders in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1 Yeast two-hybrid method to obtain positive clones and self-activation verification
[0013] Yeast two-hybrid experiments were performed using the MATCHMAKER GAL Two-Hybrid System 3 system from Clontech. The specific steps are: 1) Transform the bait plasmid (BD) containing the glucoamylase domain in human intestinal α-glucosidase into the yeast strain AH109, and prepare it into a competent state by using the PEG / LiAc method; 2) Take 10 μg of the plasmid DNA of the random eicosopeptide library was transformed into the competence of the previous step; 3) Finally, the competent cells were spread on the growth-deficient medium and cultured at 30°C until the positive clones grew; 4) The positive clones Carry out small-scale expansion culture, extract the plasmid, transfer it into AH109, and spread it on a suitable defective medium for repeated experiments and self-activation verification; 5) Send the positive plasmid without self-activation to the sequencing com...
Embodiment 2
[0015] Example 2 Chemical Peptide Synthesis of Candidate Peptide Sequences
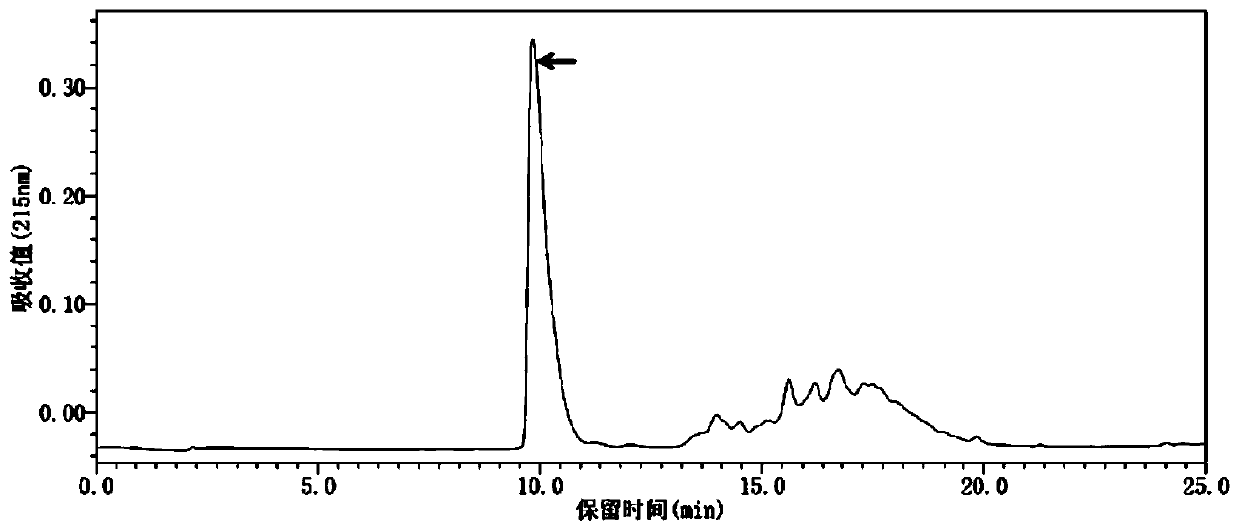
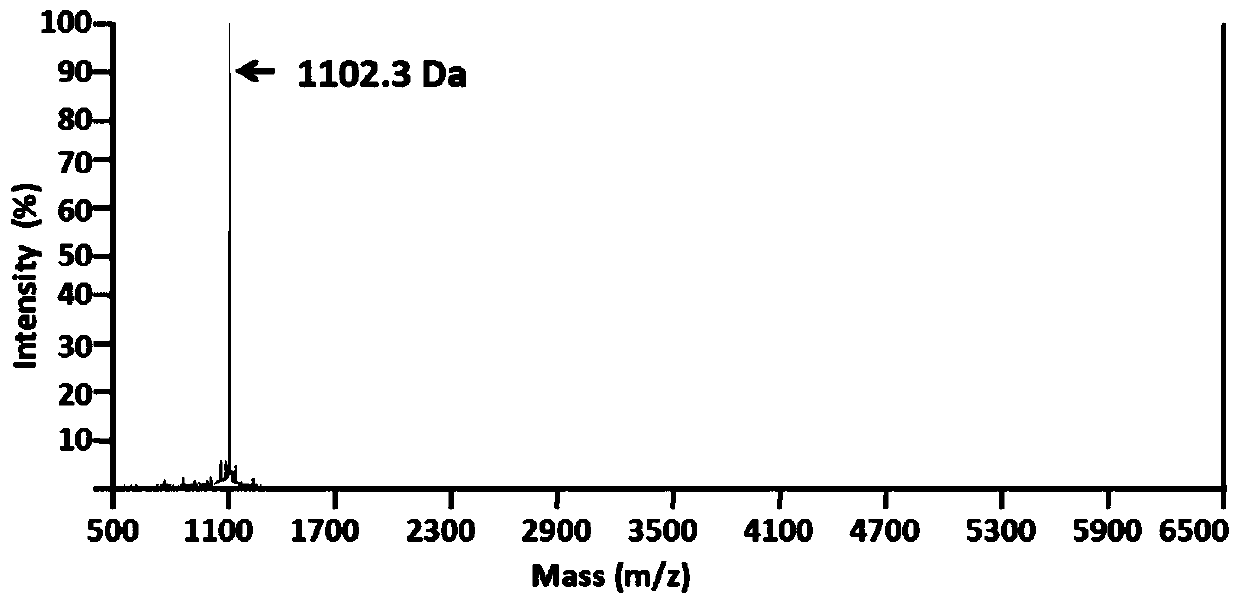
[0016] Peptides were synthesized on a CEM Liberty microwave-assisted automatic peptide synthesizer using the Fmoc protection strategy, with a microwave power of 35 W and a maximum reaction temperature of 75 °C. Rink Amide NovaPEG resin was used, 20% piperidine / DMF solution was used as deprotecting agent, peptide bond was formed by HBTU coupling, and reactants were fed according to 4 times the molar number of resin loading. After the construction of the polypeptide chain is completed, 2 g of peptide resin is obtained. The peptide resin was cleaved, TFA was removed under reduced pressure, and the crude peptide was precipitated. The crude peptide was then purified by RP-HPLC ( figure 1 RP-HPLC purification profile, acetonitrile concentration is eluted from 5%-30%), MALDI-TOF-MS analysis of the target product (see figure 2 ), freeze-drying and other steps, and finally obtain high-purity polypeptide fr...
Embodiment 3
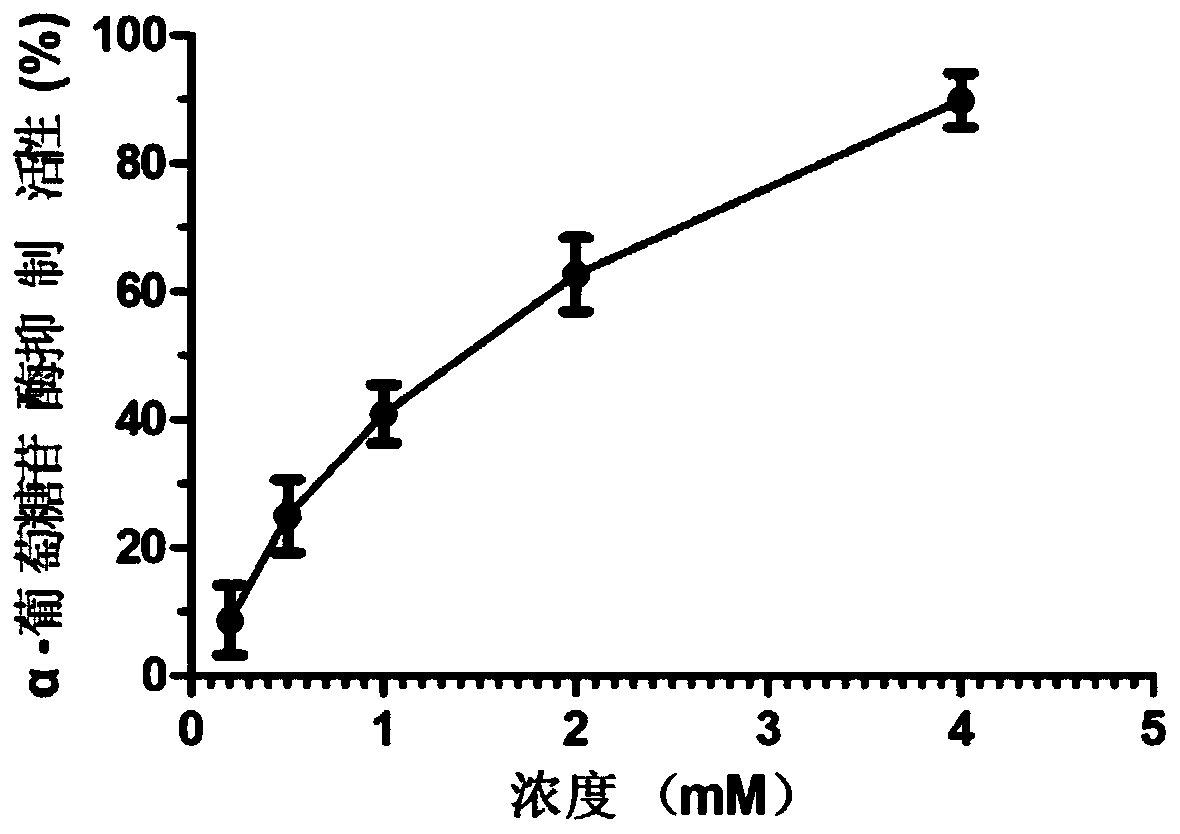
[0017] Example 3 In vitro enzyme activity test to detect the α-glucosidase inhibitory activity of the polypeptide
[0018] Add 112 μL of 0.1 mol / L PBS (pH=6.8), 20 μL of 0.2 U / mL α-glucosidase and 8 μL of peptide solution in sequence to the wells of the 96-well ELISA plate (prepare different concentrations with PBS) 2.5 mmol / L 4-nitrophenyl-α-D-glucopyranoside (PNPG), mixed and kept at 37°C for 15 min. After reacting for 15 min, add 80 μL of 0.2 mol / L Na 2 CO 3 The solution terminated the reaction, and its absorbance value at a wavelength of 405 nm was detected by a microplate reader. The experiment was repeated 3 times, and the average value was obtained.
[0019] The formula for enzyme activity inhibition rate: I%=[1-(A1-A2) / (A3-A4)]×100%; where: A1 is the absorbance value of the sample solution group, and A2 is measured by replacing the enzyme solution with PBS A3 is the absorbance value of the blank control group measured by replacing the sample solution with PBS soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com