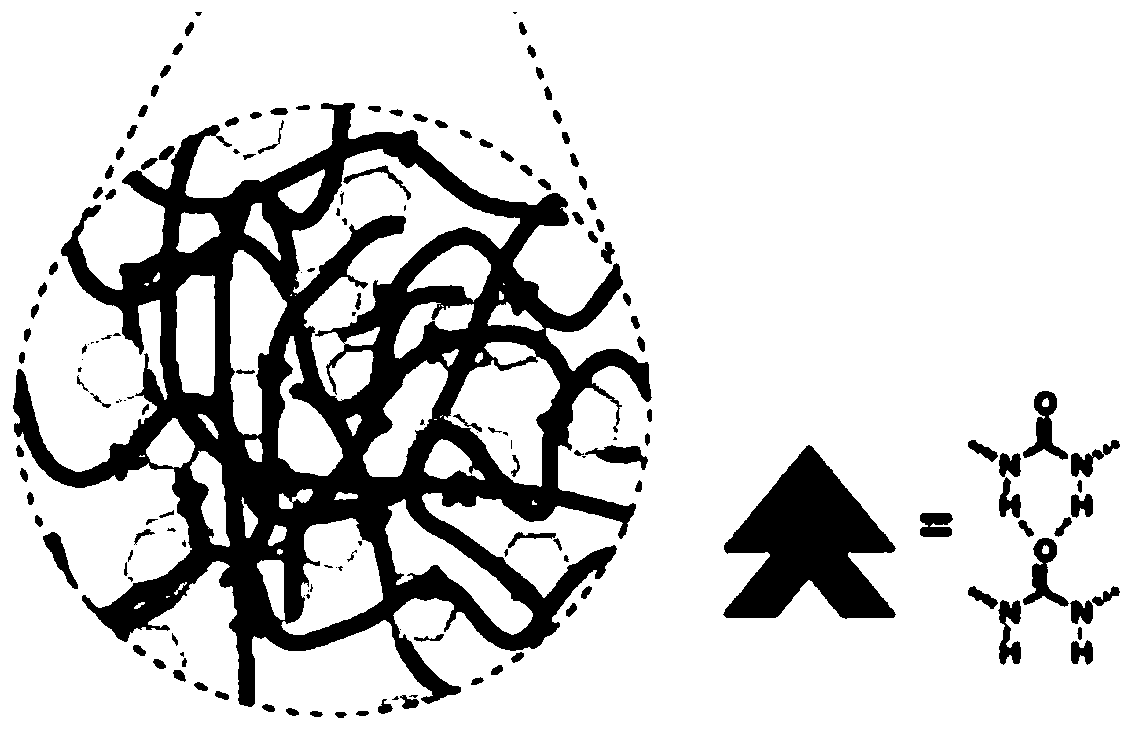
Self-repairing composite solid electrolyte, quasi-solid electrolyte and lithium battery
A solid electrolyte, composite electrolyte technology, applied in the battery field, can solve the problems of low conductivity, battery performance degradation, poor mechanical stability and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The second aspect of the present invention provides a method for preparing a self-healing composite solid electrolyte, comprising: preparing an inorganic solid electrolyte, adding the inorganic solid electrolyte into a self-healing polymer to obtain a dispersion system, and preparing it through coating and drying.
[0042] In the preparation method of the self-repairing composite solid electrolyte provided by the present invention, the preparation of the inorganic solid electrolyte includes dissolving gallium nitrate, lithium nitrate, lanthanum nitrate, and zirconium acetylacetonate in a mixed solvent of ethanol-water, and controlling the ethanol-water The volume ratio of the solution is 2:1 to 5:1, adding citric acid to fully complex the metal ions in the solution to obtain a uniform sol. The obtained sol is first heated at 60-90°C for 4 hours, then heated to 180-200°C for 8-12 hours to obtain a gel, and finally fully dried at 200-250°C to obtain a xerogel. The obtaine...
Embodiment 1
[0063] (1) Ga 0.25 Li 6.25 La 3 Zr 2 o 12 Preparation of Inorganic Solid Electrolyte
[0064] According to Ga 0.25 Li 6.25 La 3 Zr 2 o 12 The stoichiometric ratio of gallium nitrate, lithium nitrate, lanthanum nitrate, and zirconium acetylacetonate were uniformly dissolved in the mixed solvent of ethanol-water, and the content of lithium nitrate was 10% in excess to compensate for the loss of lithium source during high-temperature roasting. The volume ratio of ethanol to water is 4:1, then add citric acid to fully complex the cations in the solution to obtain a white sol, then heat at 60°C for 4 hours, then heat up to 180°C for 10 hours to obtain a gel, and The gel was fully dried under the condition of 250° C. to obtain a xerogel. The obtained xerogel was calcined in a muffle furnace at 800° C. for 5 hours to obtain an oxide solid electrolyte.
[0065] (2) Preparation of self-healing composite solid electrolyte
[0066] Take 0.3 g of the self-healing polymer obtai...
Embodiment 2
[0074] (1) Ga 0.5 Li 5.5 La 3 Zr 2 o 12 Preparation of Inorganic Solid Electrolyte
[0075] According to Ga 0.5 Li 5.5 La 3 Zr 2 o 12 The stoichiometric ratio of gallium nitrate, lithium nitrate, lanthanum nitrate, and zirconium acetylacetonate were uniformly dissolved in the mixed solvent of ethanol-water, and the content of lithium nitrate was 10% in excess to compensate for the loss of lithium source during high-temperature roasting. The volume ratio of ethanol to water is 4:1, then add citric acid to fully complex the cations in the solution to obtain a white sol, then heat at 60°C for 4 hours, then heat up to 180°C for 10 hours to obtain a gel, and The gel was fully dried under the condition of 250° C. to obtain a xerogel. The obtained xerogel was calcined in a muffle furnace at 800° C. for 5 hours to obtain an oxide solid electrolyte.
[0076] (2) Composite solid electrolyte
[0077] Take 0.3g of the self-healing polymer shown in Formula 1 and dissolve it uni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com