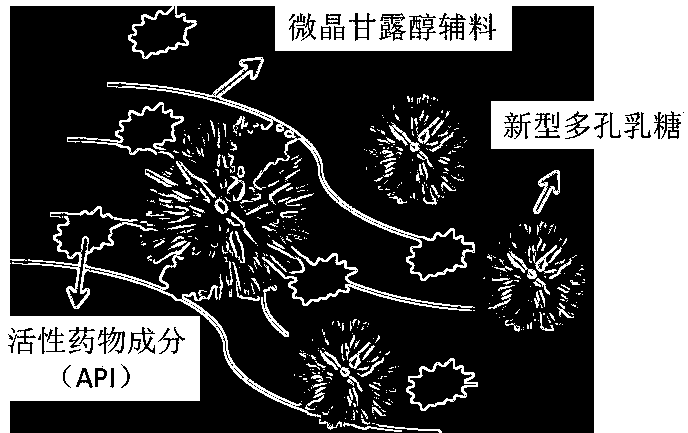
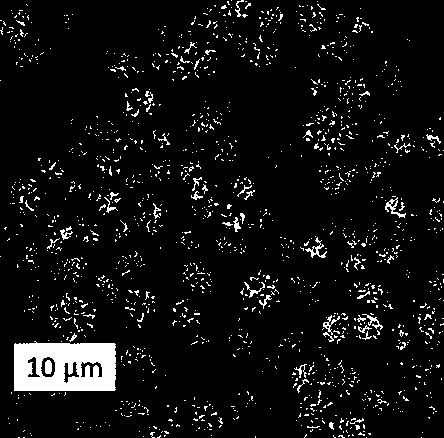
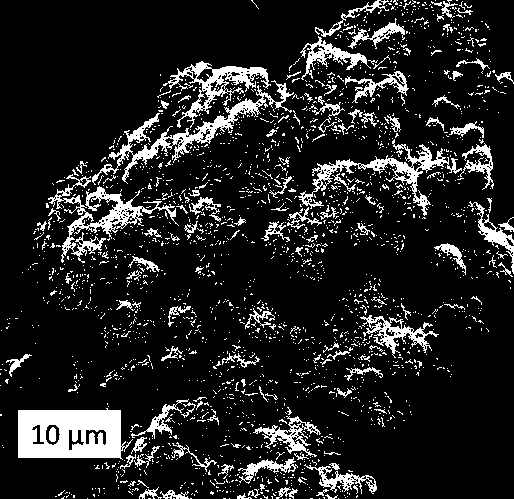
Quick-acting orally disintegrating tablet and powder based on novel porous lactose and microcrystalline mannitol auxiliary materials
A technology of alcohol excipients and mannitol, which is applied in the field of quick-acting orally disintegrating tablets and powders, can solve the problems of unfavorable safety audit and toxicology research, lack of novelty, short development history of orally disintegrating tablets, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Based on the quick-acting orally disintegrating tablet and powder of the novel porous lactose and microcrystalline mannitol excipients, this embodiment provides a preparation step of acetaminophen quick-acting orally disintegrating tablet and powder, including:
[0034] S1: Dissolve acetaminophen in ethanol solvent to obtain 22.5g / L acetaminophen ethanol solution;
[0035] S2: The amorphous lactose obtained by spray drying is added to the acetaminophen ethanol solution prepared by S1 at a concentration of 10g / L, and the novel porous lactose is obtained by crystallization, and the adsorption of the active pharmaceutical ingredients is completed;
[0036] S3: With the ratio of mannitol to ethanol of 1g:200mL (ie 5g / L), add 0.5g / mL mannitol aqueous solution to the ethanol mixed solution prepared by S2, which is a cotton-absorbent solid-ethanol mixed matrix;
[0037] S4: After the obtained mixture is dried, 0.5 g of the obtained solid is compressed into tablets or ground into powder...
Embodiment 2
[0040] Based on the quick-acting orally disintegrating tablets and powders of the novel porous lactose and microcrystalline mannitol excipients, this embodiment provides a preparation step of aspirin (also called acetylsalicylic acid) quick-acting orally disintegrating tablets and powders, including:
[0041] S1: Add the heated and dissolved 1g / mL lactose aqueous solution to the ethanol solution at a ratio of 1:50 (volume ratio) to prepare the new porous lactose, which is separated for use; with the ratio of mannitol to ethanol of 1g:100-500mL, Add 0.5 g / mL mannitol aqueous solution to the ethanol solution to prepare the microcrystalline mannitol, which is separated for use;
[0042] S2: Dissolve acetylsalicylic acid in an ethanol solvent to obtain 80g / L of an ethanol solution containing acetylsalicylic acid, and mix with 43g of new porous lactose prepared by S1 per liter to complete drug adsorption;
[0043] S3: Mix the microcrystalline mannitol made from S1 with ethanol at a ratio ...
Embodiment 3
[0047] Based on the quick-acting orally disintegrating tablet and powder of the novel porous lactose and microcrystalline mannitol excipient, this embodiment provides a preparation step of a caffeine quick-acting orally disintegrating tablet and powder, which includes:
[0048] S1: Dissolve or disperse 5g / L caffeine in an ethanol solvent to obtain an ethanol solution containing active pharmaceutical ingredients;
[0049] S2: With a ratio of mannitol to ethanol of 1g:250mL (ie 4g / L), add 0.5g / mL mannitol aqueous solution to the above ethanol mixed solution to form a cotton-absorbent solid-ethanol mixed matrix;
[0050] S3: After the obtained mixture is dried, the obtained solid is compressed into tablets or ground into powder.
[0051] The caffeine fast-acting orally disintegrating tablets and powders obtained by the above method contain ~56% by mass of the active pharmaceutical ingredient (ie API), 0% by mass of the new porous lactose, and ~44% by mass of microcrystalline mannose It s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Aperture diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com