Candida albicans applicability testing strain and preparation method thereof
A Candida albicans, applicability technology, applied in the field of Candida albicans applicability test strains and its preparation, can solve the problems of threatening the life of patients and infection caused by patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Candida albicans applicability test strain contains only one target bacterium: Candida albicans, the target concentration of the target bacterium is 500-2000 CFU / cartridge, and each bottle contains 0.2g of freeze-dried powder. The applicability test strain is based on trehalose, gelatin and sterile water, wherein the volume fraction of trehalose is 10%, and the volume fraction of gelatin is 1%.
[0030] The sample belongs to the bacterial strain used in the applicability test in drug inspection, and is used in the applicability test in the process of drug microbiological testing. The specific test process steps are as follows:
[0031] After the sample is opened, immediately add 2 mL of sterile water for rehydration, vortex and shake to mix well, take 100 μL to inoculate into the liquid medium to be tested or spread on the medium to be tested, and incubate at 30-35°C for 3-5 sky. Observe whether the growth is good, and whether the colony size and morphological charact...
Embodiment 2
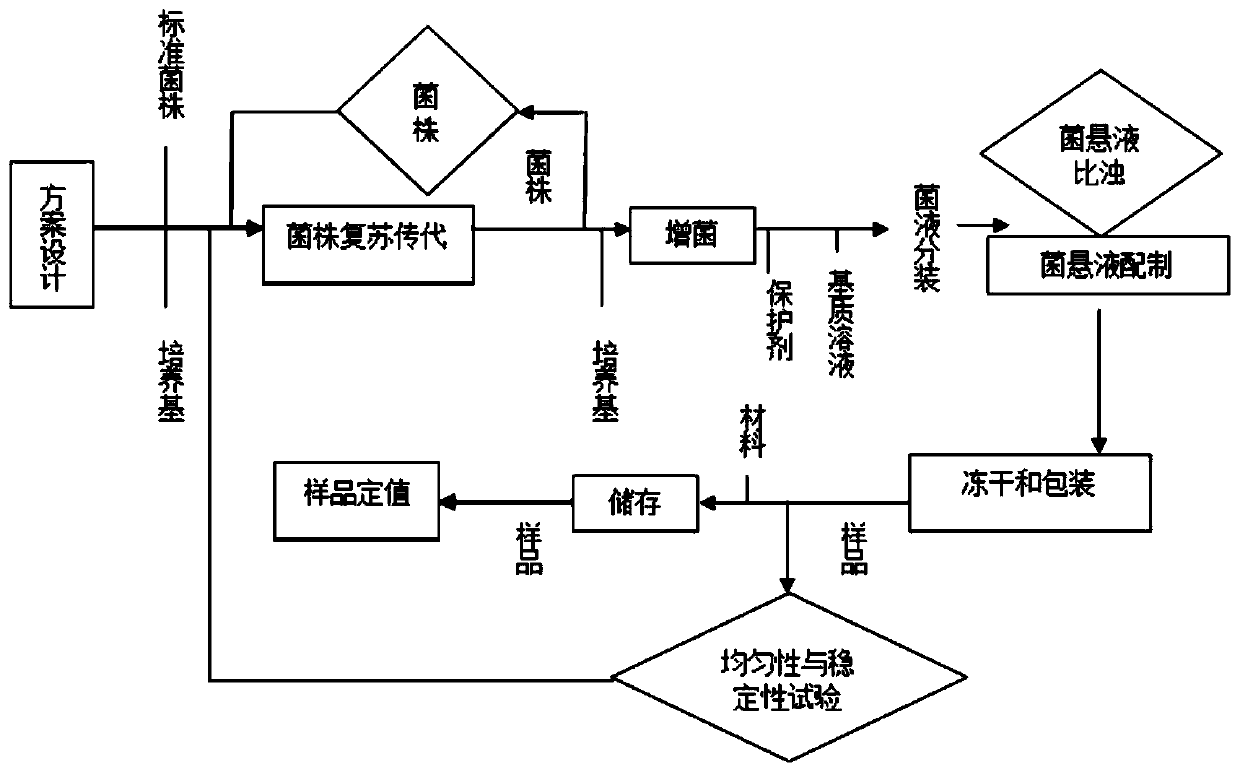
[0033] Such as figure 1 Shown, the preparation method of Candida albicans applicability test strain in a kind of embodiment 1, comprises the preparation of sample adding bacterial strain, the preparation of lyoprotectant, sample lyophilization, sample homogeneity and stability test, sample fixed value Five steps, the specific steps are as follows:
[0034] 1. Selection of sample addition strains
[0035] Contains only one target bacterium: Candida albicans, and the strains are all purchased from institutions designated by the government with strain certificates to ensure the traceability of the strains.
[0036] 2. Preparation of lyoprotectant
[0037] The sample was prepared with trehalose, gelatin and sterile water as the matrix (volume fraction: trehalose 10%, gelatin 1%) to prepare a lyoprotectant.
[0038] 3. Sample freeze-drying
[0039] (1) Strain recovery and passage
[0040] Inoculate the standard strains into nutrient agar slant medium, revive and grow them at 3...
Embodiment 3
[0081] The steps of the preparation method of the Candida albicans applicability test strain described in this example are the same as in Example 2, and the different technical parameters are: freeze-drying process 45h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com