Dual-medicine total-conveying system and making method and application thereof
A co-delivery and dual-drug technology, applied in the field of biomedicine, can solve the problems of complex preparation process and high risk of side effects, and achieve the effect of simple preparation process, excellent effect and inhibition of tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of homoharringtonine-loaded micelles
[0049] (1) Dissolve 15.0-100.0 mg of block copolymer and 5.0 mg of homoharringtonine in 4 mL of phosphate buffer, and stir to obtain polymer suspension A; the block copolymer is selected from polyethylene caprolactam-polyacetic acid Vinyl ester-polyethylene glycol (Soluplus), methoxy polyethylene glycol (mPEG)-polycaprolactone (PCL) (mPEG-PCL, molecular weight: 4000D), distearoylphosphatidylethanolamine (DSPE)- Polyethylene glycol 2000 (PEG 2000 )(DSPE-PEG 2000 , Molecular weight: 2807D), polyoxyethylene polyoxypropylene ether block copolymer (purchased from BASF, Germany, molecular weight 9840D-14600D, the content ratio of polyoxyethylene and polyoxypropylene is 80:20), polylactic acid-glycolic acid One or more of the copolymers (PLGA, 10KD-40KD, the content ratio of polylactic acid and glycolic acid is 50:50), preferably Soluplus (commercially available from BASF, Germany, molecular weight 120KD, polyethylene ...
Embodiment 2
[0057] Example 2: Load doxorubicin
[0058] (1) Dissolve 50.0-200.0 mg of the block polymer in 3 mL of phosphate buffer, stir until completely dissolved, and obtain a clear solution D;
[0059] (2) Dissolve adriamycin hydrochloride in water to prepare an aqueous solution of adriamycin hydrochloride with a concentration of 3-8 mg / mL;
[0060] (3) Mix the clear solution D with the homoharringtonine-loaded micelle C obtained in Example 1, and add 1 mL of the doxorubicin salt obtained in step (2) dropwise under heating at 25-70°C and stirring at 100-1000 r / min Stir the aqueous acid salt solution for 20-120 min to obtain solution E; the polymer of homoharringtonine micelle C is the same as the polymer used in step (1);
[0061] (4) The solution E was allowed to stand at room temperature, and filtered with a 0.22μm polyethersulfone water-based filter membrane to remove the free drug that was not encapsulated into the micelles to obtain micelle F that simultaneously contained homoharringtoni...
Embodiment 1
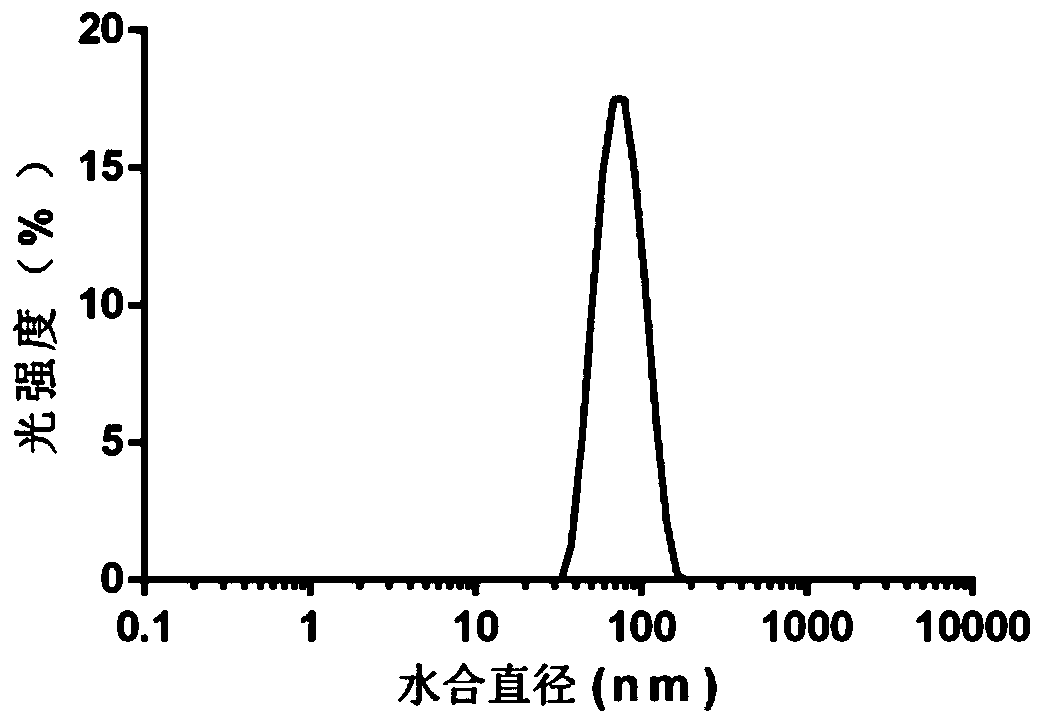
[0065] The average hydration diameter of micelle F of Example 1 is 80nm (such as figure 1 Shown), the drug loading of doxorubicin is 5%, and the encapsulation efficiency is 75%; the performance parameters of other embodiments are shown in Table 2 and Table 3.
[0066] Table 3 Dual-drug co-delivery system with different ratios of DOX and HHT
[0067]
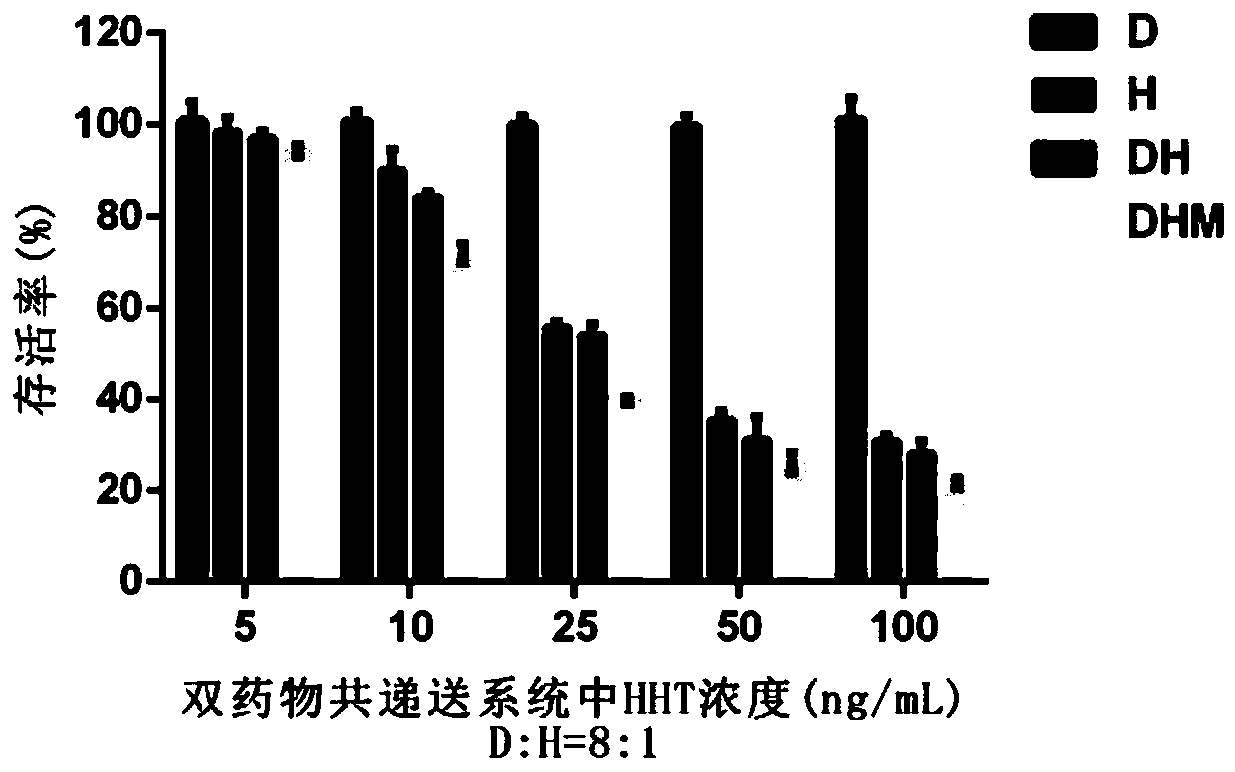
[0068] Experiment 1: Inhibition of single free drug, combination of two free drugs and dual drug co-delivery system on the proliferation of various hematological tumors and solid tumor cells in vitro
[0069] Taking HL60 / A cells (HL60 cells resistant to adriamycin) as an example, the specific implementation methods are as follows:
[0070] In Corning 96-well U-shaped plate, use 100μL RPMI-1640 medium (containing 10% fetal bovine serum and 1% penicillin) to culture 5×10 per well 4 Cells. The experimental group included: 1) Single HHT group (H): HHT was dissolved in dimethyl sulfoxide (DMSO) to obtain 10 mg / mL mother liquor; 2) Single DOX ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com