Preparation method and application of alpha-MnO2@delta-MnO2 supercapacitor electrode material
A technology for supercapacitors and electrode materials, which is applied in the manufacture of hybrid capacitor electrodes and hybrid/electric double layer capacitors. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
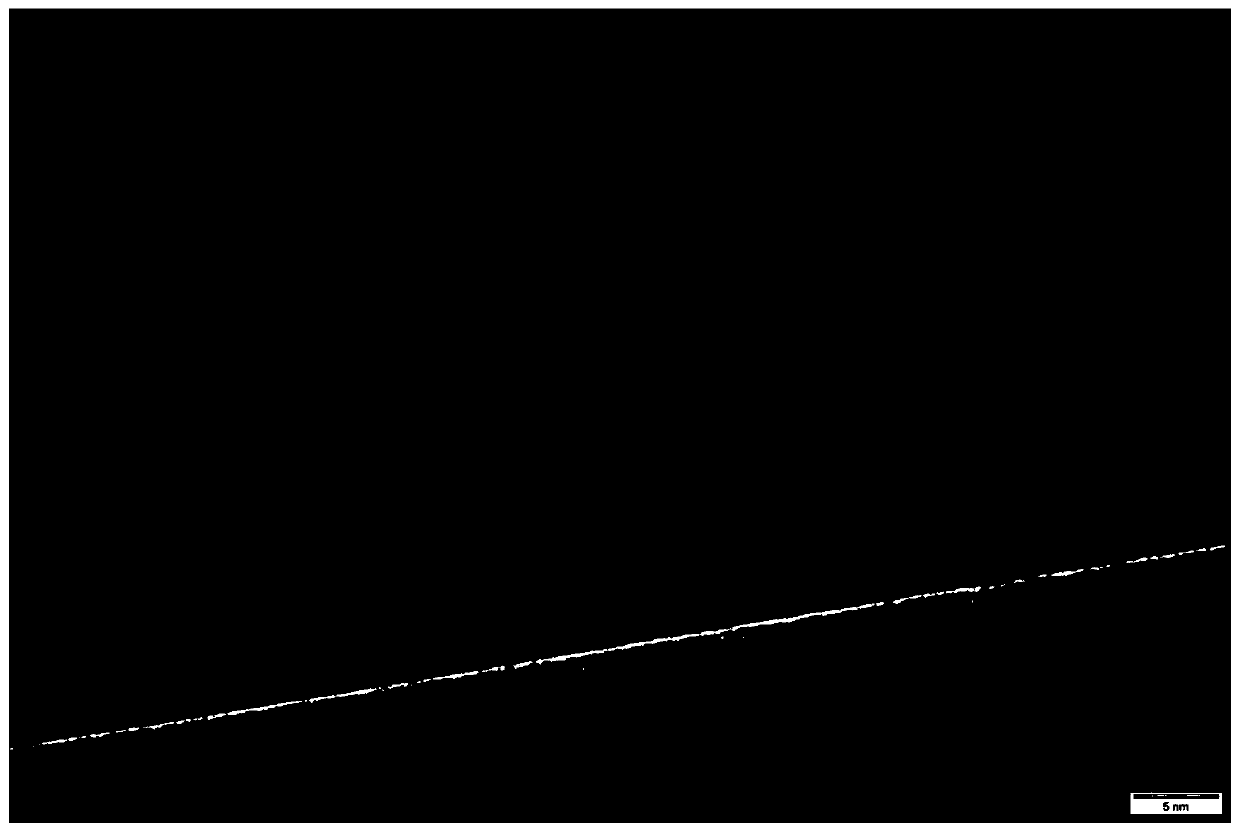
[0031] 1. Potassium permanganate (KMnO 4 ) and manganese carbonate (MnCO 3 ), add deionized water, and stir at room temperature for 30 min. Weigh 1 mmol of cobalt salt, add it into the mixture, stir evenly, and sonicate at room temperature for 20 min. The mixture was transferred to a polytetrafluoroethylene reactor and reacted at 100°C for 8h. After the reaction, cool to room temperature, centrifuge with water and ethanol several times, and dry, the resulting product is α-MnO 2 . from figure 1 It can be seen that the prepared α-MnO 2 The nanowires are uniform in length. figure 2 As can be seen in α-MnO 2 The nanowires are less than 300nm in diameter. image 3 Uniform lattice fringes indicate that the product is a single-phase material, which is consistent with α-MnO 2 The experimental results of nanowire single-phase materials are consistent. from Figure 4 It can be seen that the crystal form shows α-type MnO 2 .
[0032] 2. Potassium permanganate (KMnO 4 ) and...
Embodiment 2
[0037] 1. Potassium permanganate (KMnO 4 ) and manganese carbonate (MnCO 3 ), add deionized water, and stir at room temperature for 30 min. Weigh 3 mmol of cobalt salt, add it into the mixture, stir evenly, and sonicate at room temperature for 20 min. The mixture was transferred to a polytetrafluoroethylene reactor and reacted at 110°C for 7h. After the reaction, cool to room temperature, centrifuge with water and ethanol several times, and dry, the resulting product is α-MnO 2 . from Image 6 It can be seen that with the increase of cobalt salt doping amount, α-MnO 2 The nanowires start to bend. from Figure 7 It can be seen that the CV curves present a similar rectangular shape, indicating that the material has good electrochemical properties. Figure 8 After calculation, α-MnO 2 The specific capacitance of the nanowire is about 150F g -1 .
[0038] 2. Potassium permanganate (KMnO 4 ) and manganese carbonate (MnCO 3 ), add deionized water, and stir at room tempe...
Embodiment 3
[0040] 1. Potassium permanganate (KMnO 4 ) and manganese carbonate (MnCO 3 ), add deionized water, and stir at room temperature for 30 min. Weigh 1 mmol of cobalt salt, add it into the mixture, stir evenly, and sonicate at room temperature for 20 min. The mixture was transferred to a polytetrafluoroethylene reactor and reacted at 120°C for 6h. After the reaction, cool to room temperature, centrifuge with water and ethanol several times, and dry, the resulting product is α-MnO 2 .
[0041] 2. Potassium permanganate (KMnO 4 ) and manganese carbonate (MnCO 3 ), add deionized water, and stir at room temperature for 30 min. The α-MnO that step (1) obtains 2 Transfer to the mixed solution, further stir evenly, and sonicate for 30 min at room temperature. The mixed solution was transferred to a polytetrafluoroethylene reactor and reacted at 120°C for 6 hours. After the reaction was completed, it was cooled to room temperature, centrifuged with water and ethanol for several ti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com