Oridonin sustained release tablet and preparation method thereof
A technology of oridonin A and sustained-release tablets is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Difficult to achieve therapeutic concentration, low bioavailability and other problems, to achieve the effect of prolonging drug action time, optimizing particle fluidity and anti-caking properties, and improving sustained release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
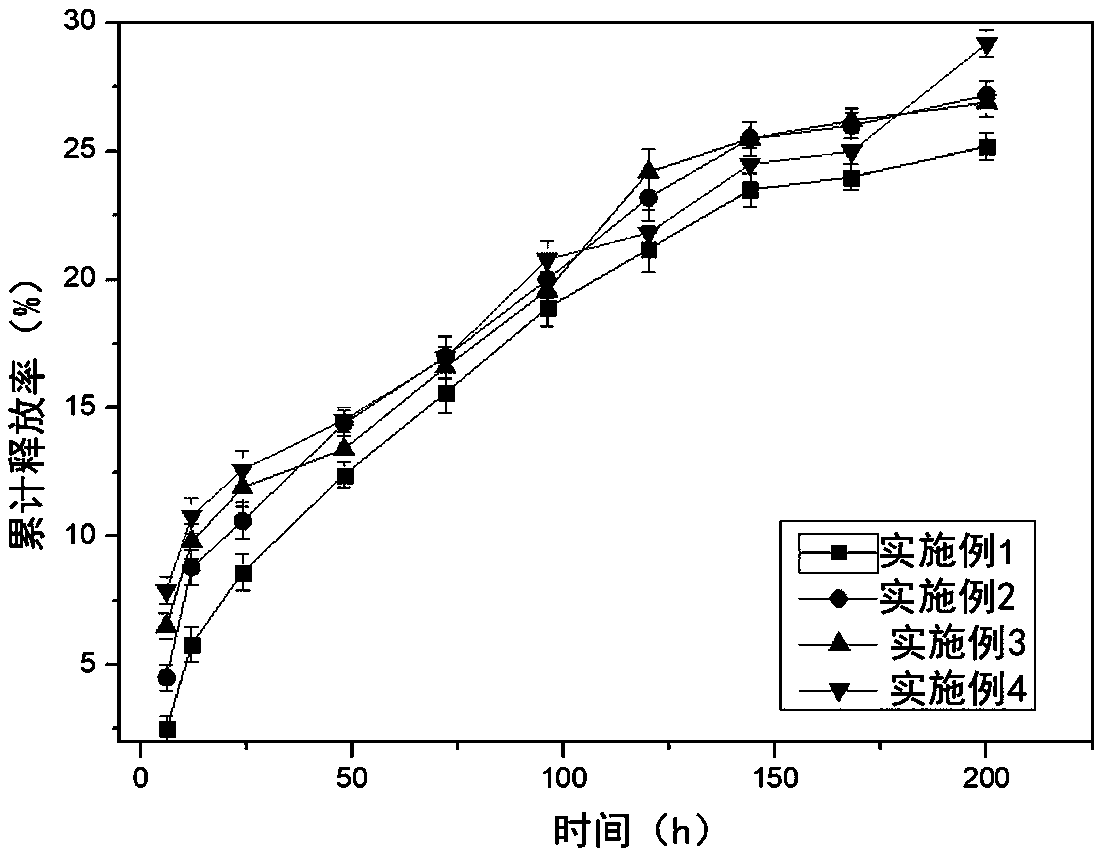
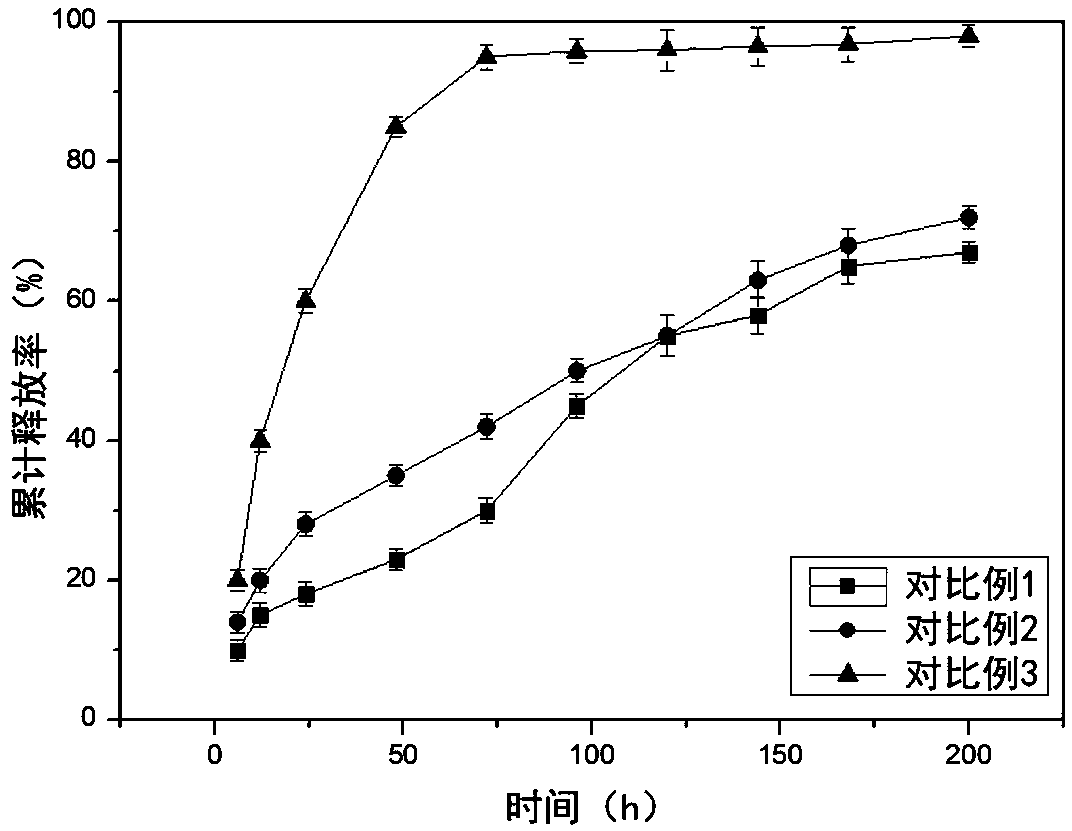
Embodiment 1
[0036] A sustained release tablet of oridonin, consisting of the following components in weight percentage: 0.3% oridonin, 30% diacylglycerol, 40% soybean lecithin, 7% chitosan, 15% ethanol, viscose Mixture 2%, low-substituted hydroxypropyl cellulose 2%, magnesium stearate 3%, sorbitol 0.7%.
[0037] The binder is formed by mixing methylcellulose and ethylcellulose in a weight ratio of 1:1-3.
[0038] This embodiment provides a preparation method of Oridonin Sustained-release Tablets, comprising the following steps:
[0039] S1. Mix the Rubescensin A in the weight percentage with phospholipids and diacylglycerol evenly, then add ethanol and mix to obtain a Rubescensin A solution;
[0040] S2, adding chitosan and adhesive to the oridonin A solution, mixing under stirring conditions, the stirring rate is 600rpm, and the stirring time is 40min, so that the above raw materials are mixed evenly;
[0041] S3. Perform high-pressure homogenization of the oridonin A cubic liquid crys...
Embodiment 2
[0046] A sustained release tablet of oridonin, consisting of the following components in weight percentage: 0.1% oridonin, 20% diacylglycerol, 50% soybean lecithin, 5% chitosan, 10% ethanol, viscose Mixture 5%, low-substituted hydroxypropyl cellulose 5%, magnesium stearate 4%, sorbitol 0.9%.
[0047] The binder is formed by mixing methylcellulose and ethylcellulose in a weight ratio of 1:1.
[0048] This embodiment provides a preparation method of Oridonin Sustained-release Tablets, comprising the following steps:
[0049] S1. Mix the Rubescensin A in the weight percentage with phospholipids and diacylglycerol evenly, then add ethanol and mix to obtain a Rubescensin A solution;
[0050] S2. Add chitosan and adhesive to the oridonin A solution, mix under stirring conditions, the stirring rate is 500rpm, and the stirring time is 1h, so that the above raw materials are mixed evenly;
[0051] S3. Perform high-pressure homogenization of the oridonin A cubic liquid crystal coarse ...
Embodiment 3
[0056] A sustained-release tablet of oridonin, consisting of the following components by weight percentage: 0.5% oridonin, 40% diacylglycerol, 30% soybean lecithin, 10% chitosan, 5% ethanol, viscose Mixture 4%, low-substituted hydroxypropyl cellulose 5%, magnesium stearate 5%, sorbitol 0.5%.
[0057] This embodiment provides a preparation method of Oridonin Sustained-release Tablets, comprising the following steps:
[0058] S1. Mix the Rubescensin A in the weight percentage with phospholipids and diacylglycerol evenly, then add ethanol and mix to obtain a Rubescensin A solution;
[0059] S2. Add the oridonin A solution to chitosan and binder, and mix under stirring conditions, the stirring rate is 800rpm, and the stirring time is 0.5h, so that the above raw materials are mixed evenly;
[0060] S3. Perform high-pressure homogenization of the oridonin A cubic liquid crystal coarse dispersion solution, the homogenization pressure is 2000 bar, and the homogenization times are 3 t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com