O-desmethylvenlafaxine phenyl ether compound and preparation method and application thereof
A technology for desmethylvenlafaxine and a compound is applied in the field of O-desmethylvenlafaxine derivatives, which can solve the problems of less research on pain uses, pain and distress of patients and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] step 1:
[0033] Add 115g of O-desvenlafaxine, 200g of 30% potassium methoxide solution, 100ml of methanol into a 2L single-necked bottle, and put it in a water bath at 85°. After reflux for 60min, remove the solvent under reduced pressure to obtain an off-white solid powder.
[0034] Step 2:
[0035] To the white solid powder obtained in step 1, add 82g of iodobenzene, 30g of cuprous iodide and 1200ml of DMF, heat the oil bath at an external temperature of 152°C under the protection of nitrogen to react for 72h, distill off most of the solvent DMF under reduced pressure, and dichloro After extraction with methane and DCM, it was washed with 2% hydrochloric acid, the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain 190 g of dark brown viscous oil.
[0036] Step 3:
[0037] Purification was carried out by column chromatography, using ethyl acetate-dichloromethane-n-heptane (1:1:1) as the elu...
Embodiment 2
[0039] Embodiment 2: in vitro experiment
[0040] Test 1: Radioligand Binding Experiment
[0041] 1.1 Experimental cells: CHO cells
[0042] 1.2 Experimental drugs: compound of formula I, nisoxetine, BTCP, imipramine, desipramine
[0043] 1.3 Experimental design:
[0044] To determine the binding of LPM580098 to serotonin transporter (SERT), norepinephrine transporter (NET) or dopamine transporter (DAT), respectively, radioligand binding experiments were performed.
[0045] 1.4 Experimental steps:
[0046] CHO cell membrane homogenate was mixed with [3H] Nisoxetine or [3H] BTCP was incubated at 4°C for 120 min, or with [3H]Imipramine was incubated at 22°C for 60 min. In this experiment, only 10 μM of LPM580098 was tested, and the non-specific binding of the compound was determined in the presence of 1 μM desipramine, 10 μM BTCP and 10 μM imipramine, respectively. Reactions were performed by rapid filtration under vacuum using glass fiber filters pre-soaked with 0.3% po...
Embodiment 3
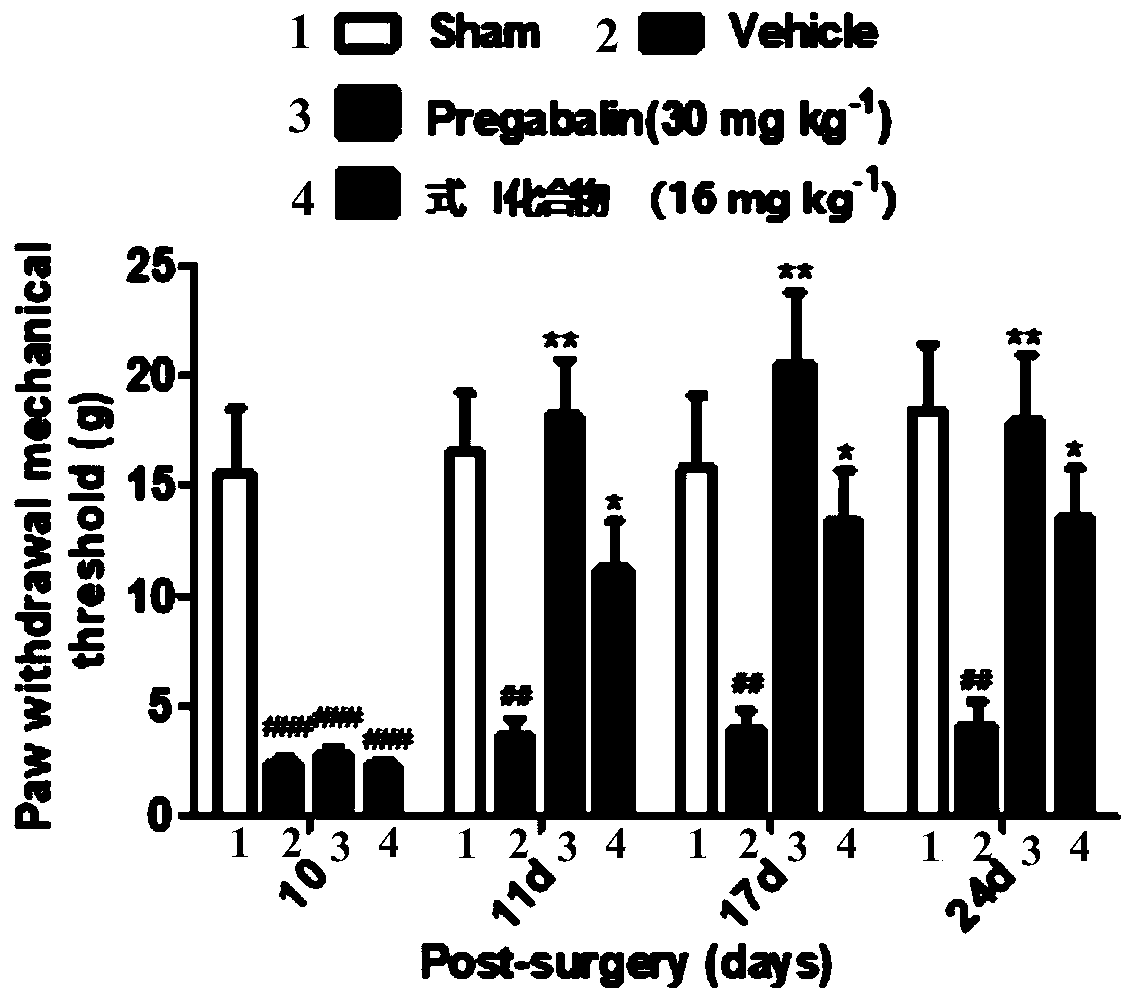
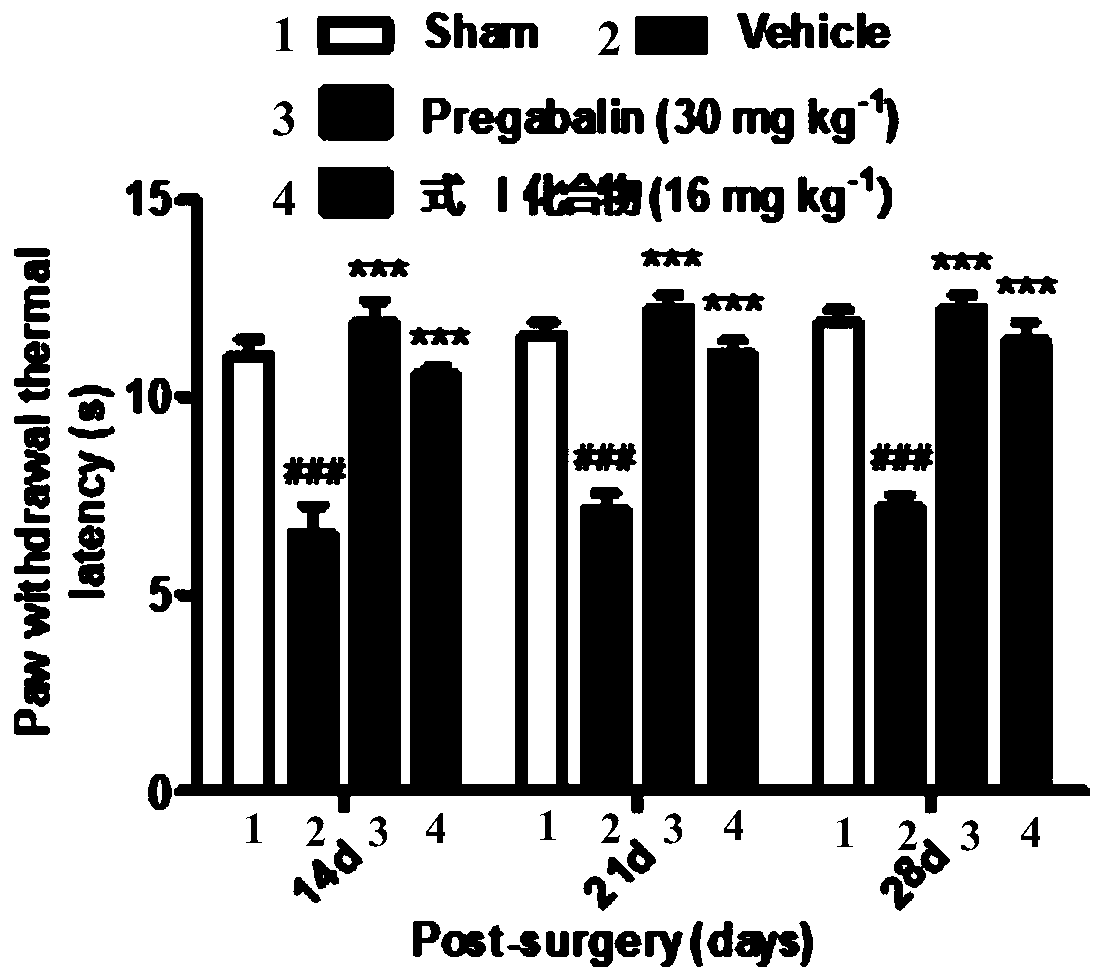
[0056] Embodiment 3: experiment in vivo
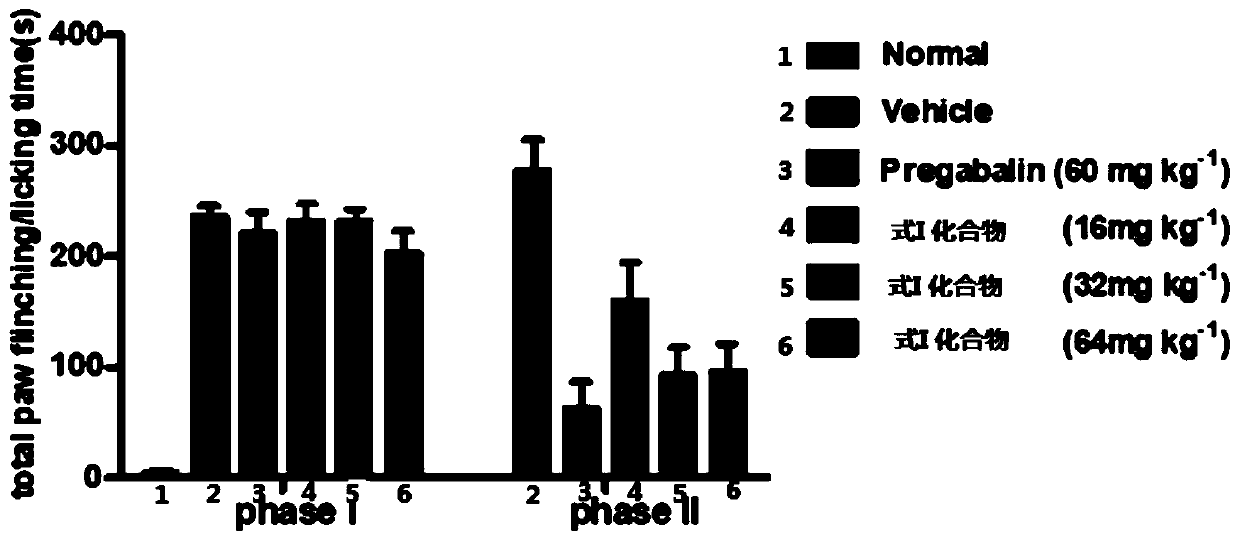
[0057] Test 1: Mouse formalin pain model
[0058] 1.1 Experimental animal: KM mouse, 18-22g
[0059] 1.2 Experimental drugs and reagents: compound of formula I, pregabalin, CMC-Na solution, formaldehyde solution
[0060] 1.3 Experimental Design: The experiment is divided into 6 groups:
[0061] 1, normal group; 2, model group; 3, pregabalin group (60mg / kg); 4, formula I compound (16mg / kg); 5, formula I compound (32mg / kg); 6, formula I compound ( 64mg / kg).
[0062] 1.4 Experimental steps:
[0063] 1.4.1 Mice were administered orally for 1 hour in advance;
[0064] 1.4.2 Put the mouse to be tested into a transparent mouse cage, and start modeling after 5 minutes of adaptation: insert a 25ul micro-syringe into the middle toe of the mouse's right hind foot, and inject 20 μL of 2.5% formalin when the needle reaches the center of the foot Lin solution (the normal group was injected with 20 μL of normal saline in the same way), and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com