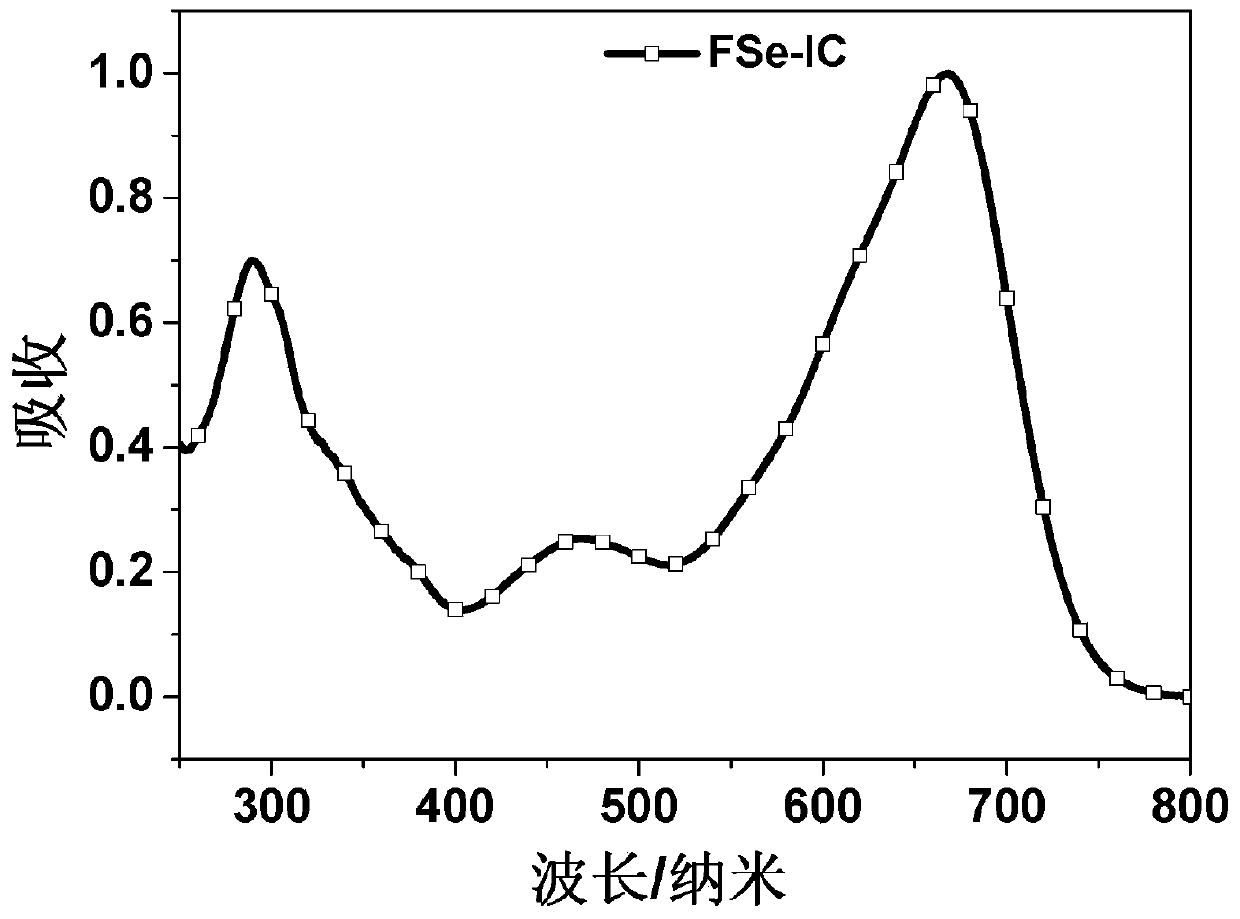
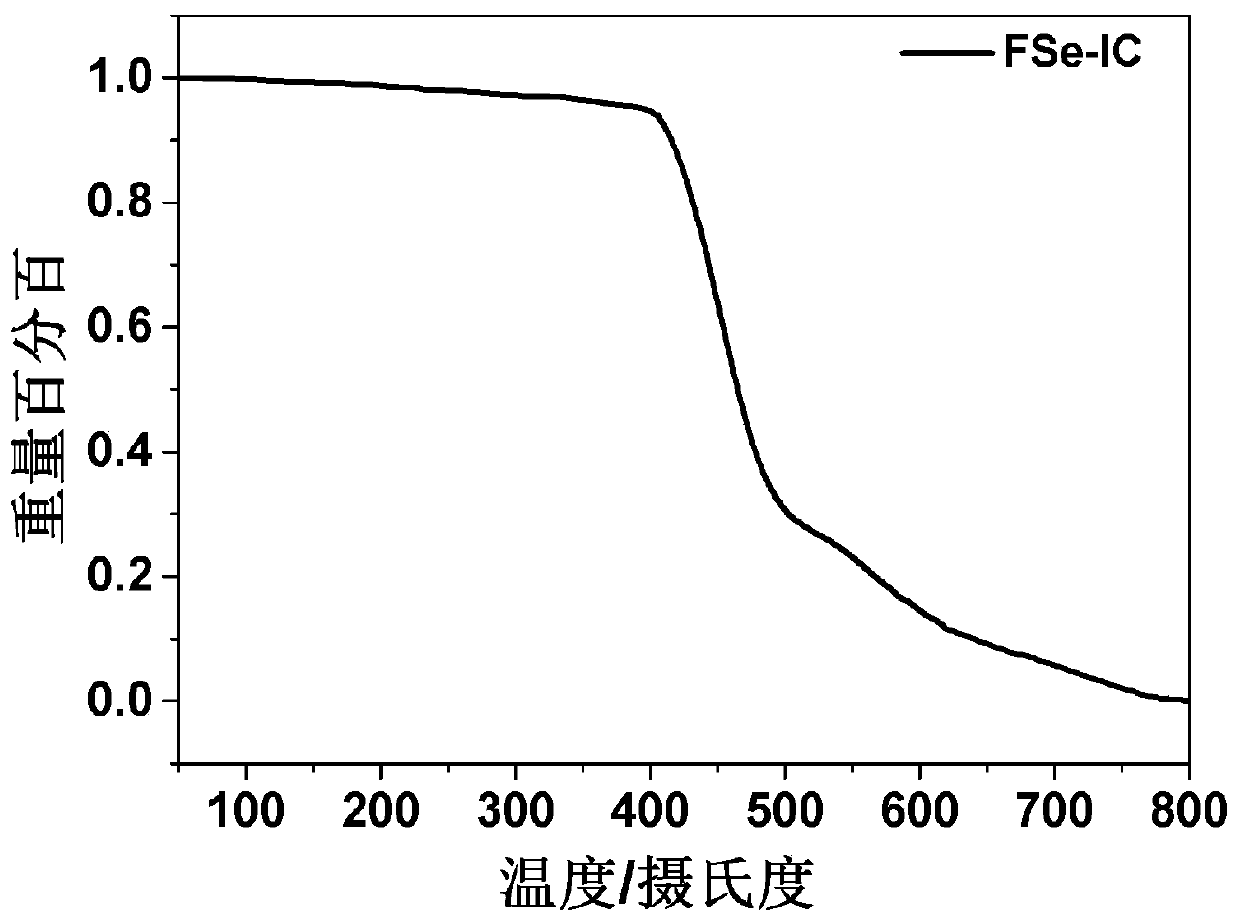
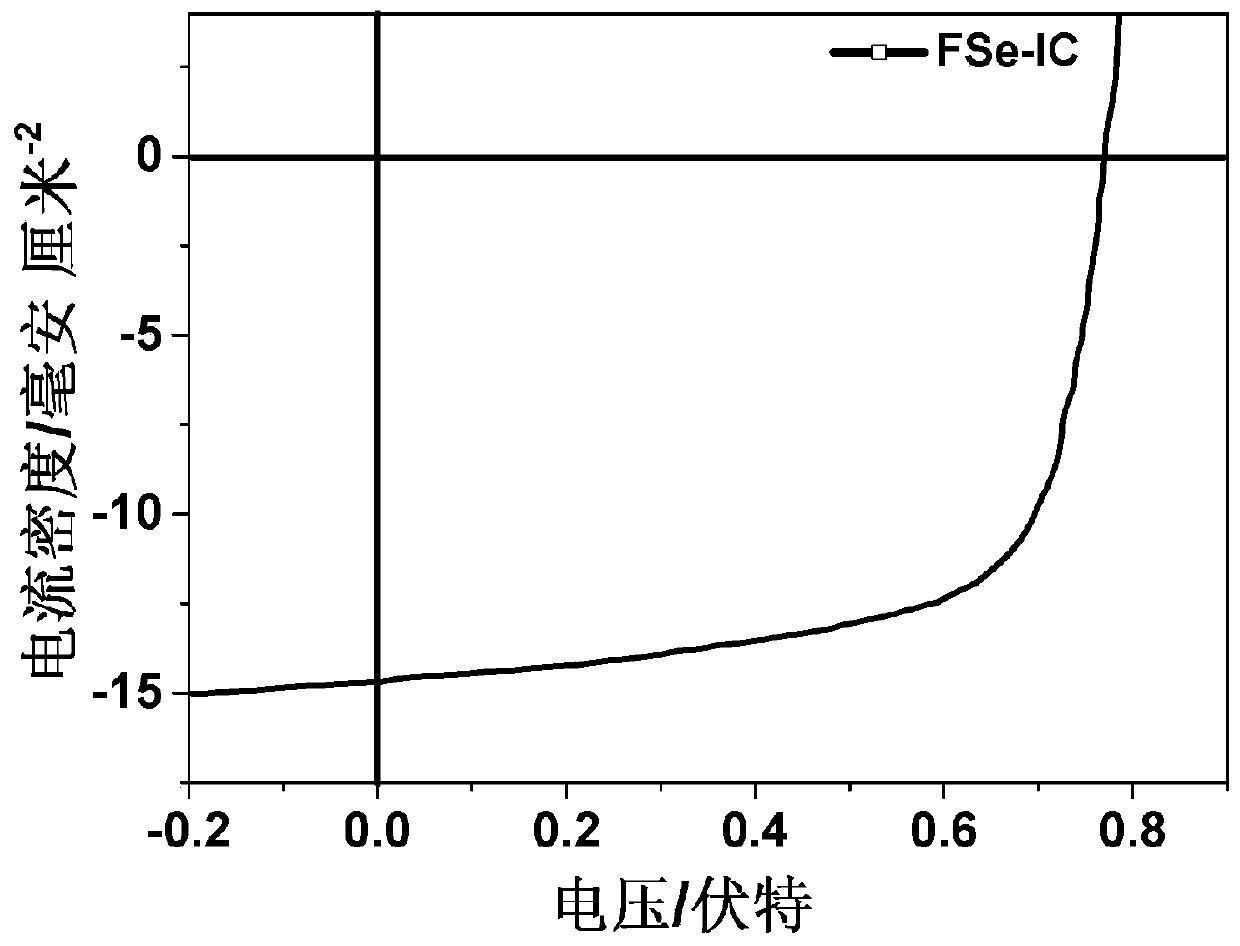
Conjugated molecular compound based on asymmetric selenium fused heterocycle and preparation method thereof
A conjugated molecule, asymmetric technology, used in organic chemistry, semiconductor/solid-state device manufacturing, photovoltaic power generation, etc., can solve the problems of difficult synthesis of central core structure and no literature reports, and achieve novel molecular structure and thermal stability. The effect of good, high charge transport properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The synthetic route of embodiment 1. compound 3 is as follows:
[0037]
[0038] Asymmetric bis-nitrated fluorene (5.20 g, 8.93 mmol) and tributyltin-based thiophene (8.33 g, 22.32 mmol) with a molar ratio of 1:2.5 in 5% mol of Pd[PPh 3 ] 2 Cl 2 (313.4mg, 0.446mmol) was catalyzed by coupling reaction in 50mL of anhydrous tetrahydrofuran solution to obtain compound 1 (4.0g, 6.79mmol). Compound 1 (2.54g, 4.32mmol) and Se (6.83g, 86.45mmol) with a molar ratio of 1:20 were closed in 80mL N-methylpyrrolidone solution to obtain an asymmetric fused ring compound 2 (2.18g, 3.34 mmol ). Compound 2 (0.884g, 1.35mmol) was reacted with phosphorus oxychloride (4.15g, 27.09mmol) and 5mL of N,N-dimethylformamide to obtain asymmetric fused ring dialdehyde compound 3 (767mg, 1.08mmol ). 1 H NMR(500MHz,DMSO)δ9.91(s,1H),9.88(s,1H),8.11(s,1H),8.08(s,1H),8.05(d,J=3.9Hz,1H),7.83 (d,J=3.9Hz,1H),7.43(s,1H),7.33(s,1H), 2.12-1.99(m,4H),1.09-0.93(m,12H),0.68(t,J=6.9 Hz,6H),0.55(s,4H).MS(M...
Embodiment 2
[0039] Embodiment 2. The synthetic route of compound FSe-IC is as follows:
[0040]
[0041] The asymmetric fused ring dialdehyde compound 3 (100mg, 0.141mmol) and the electron-withdrawing unit 3-(dicyanomethylene)inden-1-one (134mg, 0.691mmol) at a molar ratio of 1:5 were prepared in the absence of In an oxygen-free reaction vessel, use chloroform as a solvent, add 0.2 mL of pyridine, heat and reflux for 6 hours, pour the product into methanol solution after the reaction, and purify the filtered solid product by column chromatography to obtain an asymmetric selenium complex Ring Conjugated Molecular Compound FSe-IC (109mg, 0.103mmol). 1 HNMR (500MHz, CDCl3) δ8.94 (s, 1H), 8.90 (s, 1H), 8.74 (d, J = 7.3Hz, 1H), 8.70 (d, J = 7.3Hz, 1H), 8.10 (s, 2H), 7.94-7.88(m,4H),7.83-7.78(m,4H),7.64(s,1H),7.45(s,1H),2.07(m,4H), 1.16-1.01(m,12H) ,0.79-0.66(m,10H).MS(MALDI):m / z 1060.56.Anal.Calcd for C 59 h 42 N 4 o 2 S 2 Se 2 : C, 66.79; H, 3.99. Found: C, 66.56; H, 4.04%.
[004...
Embodiment 3
[0043] Embodiment 3. The synthetic route of compound FSe-IC is as follows:
[0044]
[0045] The asymmetric fused ring dialdehyde compound 3 (150mg, 0.212mmol) with a molar ratio of 1:5 and the electron-withdrawing unit 5,6-difluoro-3-(dicyanomethylene)inden-1-one ( 239mg, 1.04mmol) in an oxygen-free anhydrous reaction vessel, with chloroform as solvent, add 0.2mL of pyridine, heat and reflux for 6 hours, pour the product into methanol solution after the reaction, and filter the solid product obtained by column chromatography The asymmetric selenium heterofused ring conjugated molecular compound FSe-2FIC (191mg, 0.168mmol) was obtained after purification. MS(MALDI):m / z 1133.56.Anal.Calcd for C 59 h 38 f 4 N 4 o 2 S 2 Se 2 : C, 62.55; H, 3.38. Found: C, 62.96; H, 3.24%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com