Solid base catalyst and preparation method and application thereof
A solid base catalyst and carrier technology, applied in the field of catalysis, to achieve the effects of harsh reaction conditions, high and low temperature catalytic activity and product selectivity, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation method of solid base catalyst comprises the steps:
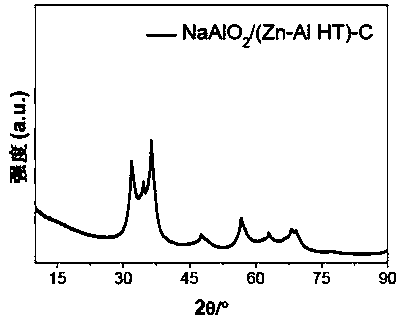
[0047] Prepare 100mL of mixed aqueous salt solution of zinc nitrate and aluminum nitrate with deionized water, wherein the concentration of zinc ions is 0.6mol / L and the concentration of aluminum ions is 0.3mol / L; prepare 80mL of mixed alkali of sodium hydroxide and sodium carbonate Solution, wherein, sodium hydroxide concentration is 2.0mol / L, and sodium carbonate concentration is 0.5mol / L. After stirring the above mixed salt solution and mixed alkali solution for 1 hour, drop them into a three-neck flask at 75°C, control the pH to 9.5, stir thoroughly for 1.5 hours, and transfer the slurry to a 200mL stainless steel reaction vessel lined with polytetrafluoroethylene. Crystallize at 120°C for 4 hours in a kettle, wash the resulting slurry with deionized water to a pH range of 7-8, then dry at 90°C for 24 hours, and grind to prepare a zinc-aluminum hydrotalcite precursor. Prepare sodium aluminate into...
Embodiment 2
[0049] The preparation method of solid base catalyst comprises the steps:
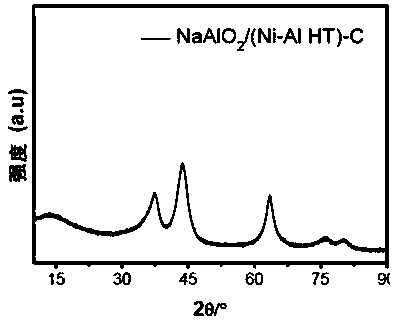
[0050] Prepare 100mL of mixed aqueous salt solution of nickel nitrate and aluminum nitrate with deionized water, wherein the concentration of nickel ions is 1.2mol / L, and the concentration of aluminum ions is 0.4mol / L; prepare 80mL of mixed alkali of sodium hydroxide and sodium carbonate Solution, wherein, sodium hydroxide concentration is 2.0mol / L, and sodium carbonate concentration is 0.5mol / L. After stirring the above mixed salt solution and mixed alkali solution for 1 hour, drop them into a three-neck flask at 75°C, control the pH to 10, stir thoroughly for 1.5 hours, and transfer the slurry to a 200mL stainless steel reaction vessel lined with polytetrafluoroethylene. Crystallize at 120°C for 4 hours in a kettle, wash the resulting slurry with deionized water to a pH range of 7-8, then dry at 90°C for 24 hours, and grind to prepare a nickel-aluminum hydrotalcite precursor. Prepare sodium aluminat...
Embodiment 3
[0052] The preparation method of solid base catalyst comprises the steps:
[0053] Prepare 100 mL of a mixed aqueous salt solution of cobalt nitrate and aluminum nitrate with deionized water, wherein the concentration of cobalt ions is 0.6 mol / L and the concentration of aluminum ions is 0.2 mol / L; prepare 80 mL of mixed alkali of sodium hydroxide and sodium carbonate Solution, wherein, sodium hydroxide concentration is 2.0mol / L, and sodium carbonate concentration is 0.5mol / L. After stirring the above mixed salt solution and alkali solution for 1 hour, drop them into a three-neck flask at 75°C, control the pH to 10, stir thoroughly for 1.5 hours, and transfer the slurry to a 200mL stainless steel reaction kettle lined with polytetrafluoroethylene , crystallized at 140°C for 4 hours, washed the resulting slurry with deionized water to a pH range of 7-8, then dried at 90°C for 12 hours, and ground to obtain the cobalt aluminum hydrotalcite precursor. Prepare sodium aluminate int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com