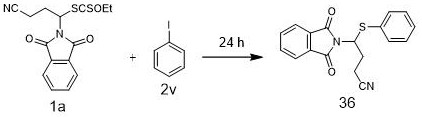
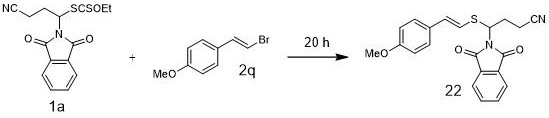
A kind of method and application of synthesizing organosulfur compound based on alkyl xanthate
A technology of alkyl xanthate and organic sulfur, which is applied in the direction of organic chemistry, organic chemistry, chemical instruments and methods, etc., can solve the problem of harsh reaction conditions for the synthesis of sulfur compounds, poor compatibility of functional groups, and low total yield of reactions, etc. problems, to achieve the effect of easy access, low cost and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
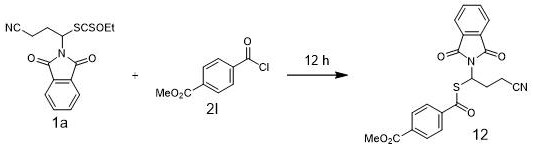
[0032] Under nitrogen protection, nickel chloride ethylene glycol dimethyl ether complex (0.05 mmol), 6,6'-dimethyl-2,2'-bipyridine (0.05 mmol) were sequentially added to a 25 mL reaction flask, Alkyl xanthate 1a (0.5 mmol), electrophile 2a (0.6 mmol), N,N-dimethylpropenylurea (2.5 mL), potassium iodide (0.25 mmol) and elemental zinc (1.5 mmol). After mixing uniformly at room temperature, the reaction mixture was reacted at 40 °C for 12 h. After the reaction was completed, 5 mL of water was added, extracted with ethyl acetate (5 mL×3), the organic phases were combined, the solvent was evaporated under reduced pressure, and then separated by column chromatography (petroleum ether:ethyl acetate V / V=10:2) to obtain Compound 1, IR (neat): 2360, 1780, 1714, 1468, 1207, 901, 773, 716, 684, 646. 1 HNMR (400 MHz, CDCl 3 ): δ 7.98 – 7.91 (m, 2H), 7.91 – 7.84 (m, 2H), 7.80 – 7.73(m, 2H), 7.65 – 7.55 (m, 1H), 7.52 – 7.41 (m, 2H), 6.40 – 6.30 (m , 1H), 2.82 – 2.67 (m, 1H)...
Embodiment 2
[0034]
[0035] Under nitrogen protection, nickel bromide ethylene glycol dimethyl ether complex (0.05 mmol), 1,10-phenanthroline (0.05 mmol), and alkyl xanthate 1a (0.5 mmol) were sequentially added to a 25 mL reaction flask. mmol), electrophile 2b (0.7 mmol), N,N-diacetamide (2.5 mL), (Boc) 2 O (1.4 mmol), magnesium chloride (0.7 mmol) and elemental zinc (1.5 mmol). After mixing uniformly at room temperature, the reaction mixture was reacted at 40 °C for 10 h. After the reaction was completed, 5 mL of water was added, extracted with ethyl acetate (5 mL×3), the organic phases were combined, the solvent was evaporated under reduced pressure, and then separated by column chromatography (petroleum ether:ethyl acetate V / V=10:2) to obtain Compound 2, IR (neat): 2920, 2360, 2342, 1760, 1377, 1084, 882, 750, 717, 528. 1 HNMR (400 MHz, CDCl 3 ): δ 7.87 (m, 2H), 7.79 – 7.70 (m, 2H), 7.27 (m, 1H), 7.24(s, 1H), 7.16 (m, 3H), 6.10 (m, 1H), 2.69 – 2.54 (m, 5H), 2.54 – 2.35 (m, 3H...
Embodiment 3
[0037]
[0038] Under nitrogen protection, nickel chloride ethylene glycol dimethyl ether complex (0.05 mmol), 6,6'-dimethyl-2,2'-bipyridine (0.05 mmol) were sequentially added to a 25 mL reaction flask, Alkyl xanthate 1a (0.5 mmol), Electrophile 2c (0.7 mmol), N,N-Dimethylpropenylurea (2.5 mL), (Boc) 2 O (1.4 mmol), magnesium chloride (0.7 mmol) and elemental zinc (1.5 mmol). After mixing uniformly at room temperature, the reaction mixture was reacted at 40 °C for 12 h. After the reaction was completed, 5 mL of water was added, and extracted with ethyl acetate (5 mL×3), the organic phases were combined, the solvent was evaporated under reduced pressure, and then separated by column chromatography (petroleum ether:ethyl acetate V / V=10:2) to obtain the compound 3. IR (neat): 3853, 3675, 2987, 2360, 2342, 1717, 1559, 1395, 1066, 669. 1 H NMR (400 MHz, CDCl 3 ): δ 10.11 (s, 1H), 8.08 (d, J = 8.2 Hz, 2H), 7.96 (d, J = 8.3 Hz, 2H), 7.93 – 7.86 (m, 2H), 7.83 – 7.73 (m, 2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com