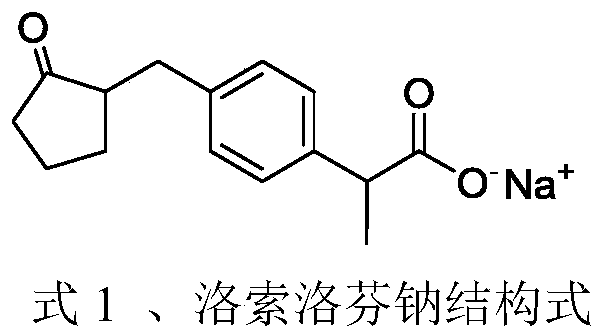
Synthesis method of loxoprofen sodium
A technique for loxoprofen sodium and a synthetic method, which is applied in the chemical industry, can solve problems such as high cost and low yield, and achieve the effects of low production cost, increased product yield, and reduced reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
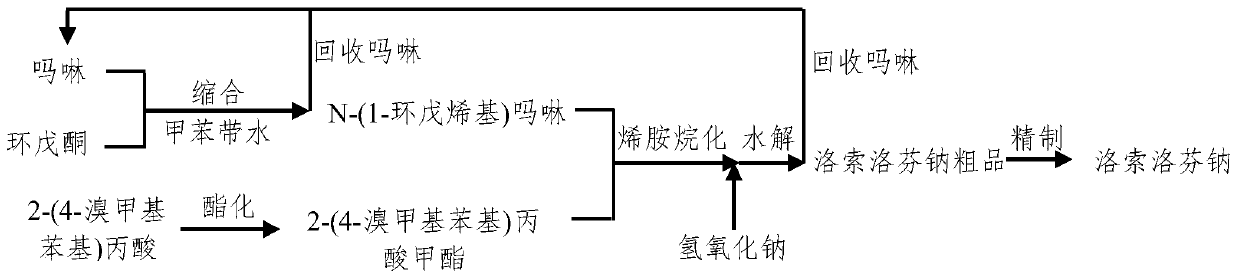
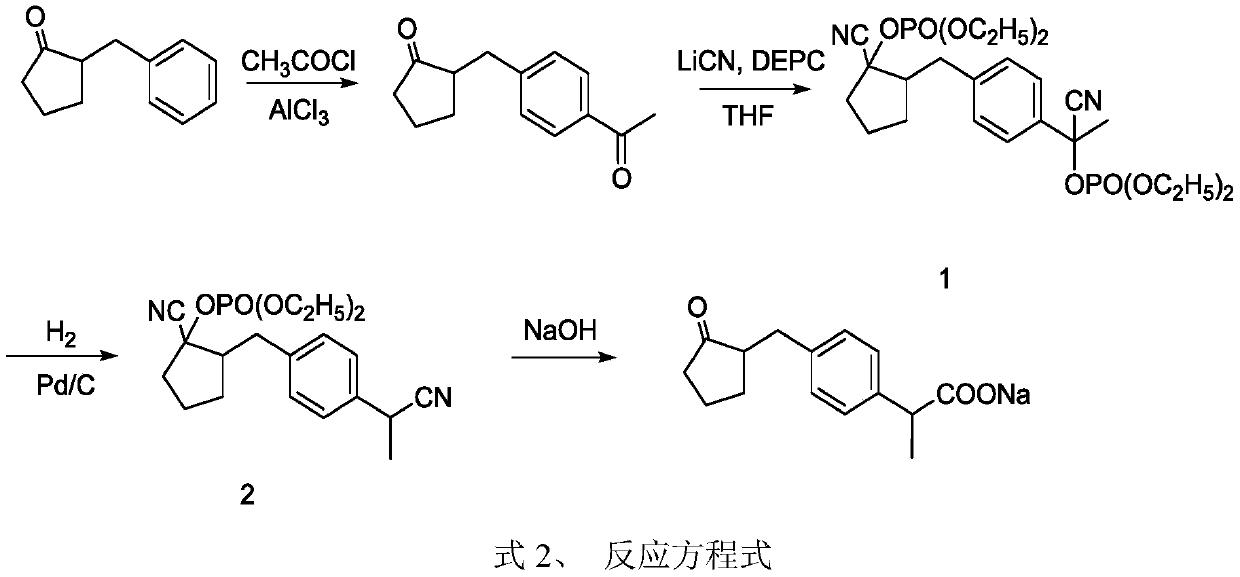
[0050] The synthetic method of embodiment 1, loxoprofen sodium, carries out following steps successively:
[0051] 1), condensation reaction prepares N-(1-cyclopentenyl) morpholine:
[0052] Take 16.8g (0.2mol, 18ml) of cyclopentanone and 52.3g (0.6mol) of morpholine into a 250mL three-neck flask, add 0.2g of p-toluenesulfonic acid, 100mL of toluene, heat to reflux (about 110°C), and azeotrope With water, remove the water generated by the reaction from the reaction system in real time to promote the reaction. After 8 hours of reaction, 3.4g of water is brought out; at this time, no more water is taken out; the resulting reaction solution is distilled at atmospheric pressure (110-130°C) , Collect about 116.6g of the preceding cut, then vacuum distillation, collect the cut (20mmHg) of 108~115 ℃, obtain product N-(1-cyclopentenyl) morpholine 27.3g, yield is 89.3%.
[0053] The main components of the former fraction are toluene and morpholine, of which morpholine contains about 3...
Embodiment 6
[0072] The morpholine used in embodiment 1 step 1) is changed into the morpholine used in embodiment 1 step 4) and the molar consumption remains unchanged; the toluene used in step 1) and step 3) is all changed into embodiment 1 step 4) reclaim the toluene of gained, volume consumption remains unchanged; All the other are equal to embodiment 1.
[0073] The yield of loxoprofen obtained in step 3) is 77.2%.
Embodiment 7
[0074] Embodiment 7, change the consumption of sodium hydroxide aqueous solution in embodiment 1 step 3), thereby change the mol ratio of sodium hydroxide and N-(1-cyclopentenyl) morpholine; All the other are equal to embodiment 1; Process parameter Shown in table 2 with the contrast of result and embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com