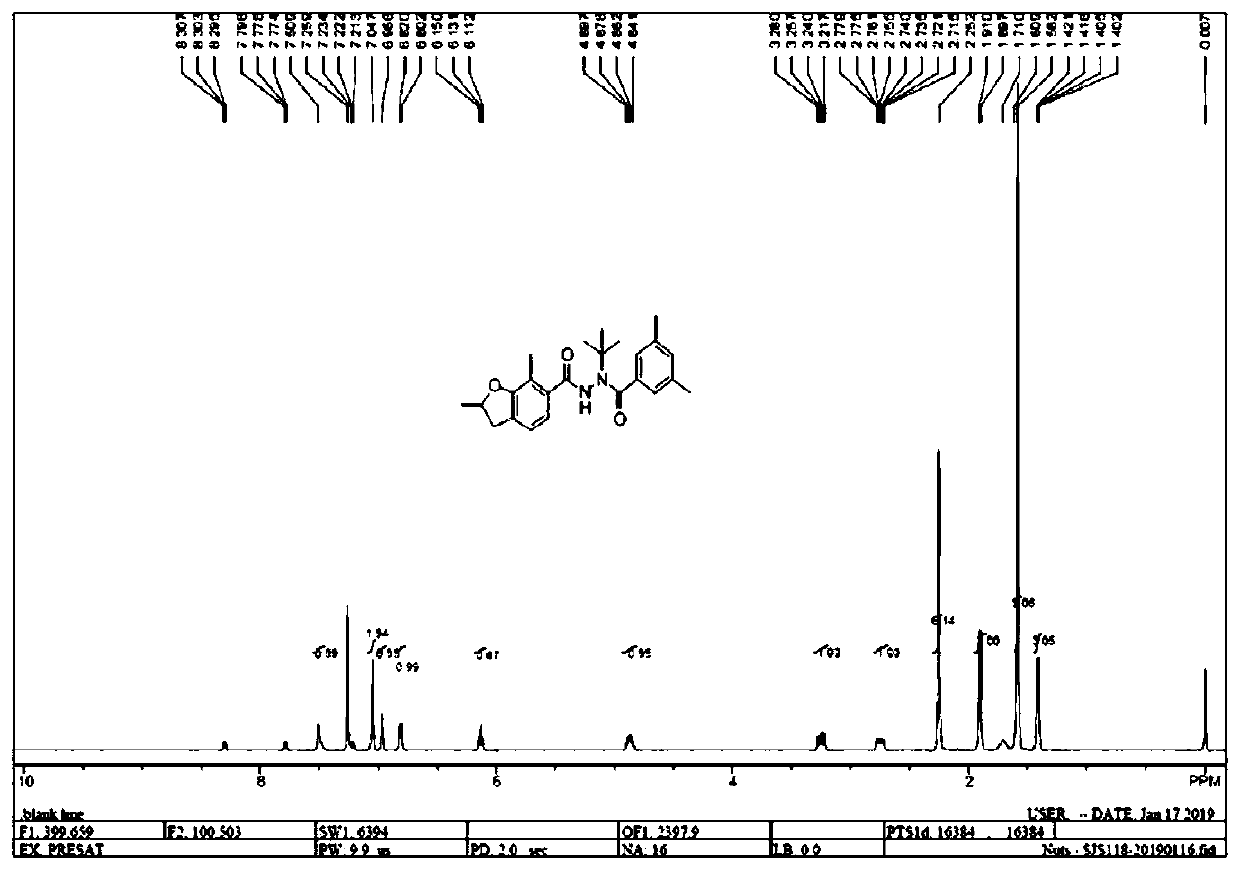
Preparation method of furantefenozide, intermediate and preparation method thereof
A technology of furanfenozide and intermediates, which is applied in the field of pesticide compounds, can solve the problems of high cost, low reaction selectivity, and low yield, and achieve the effects of low total cost, good reaction selectivity, and safe and reliable reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
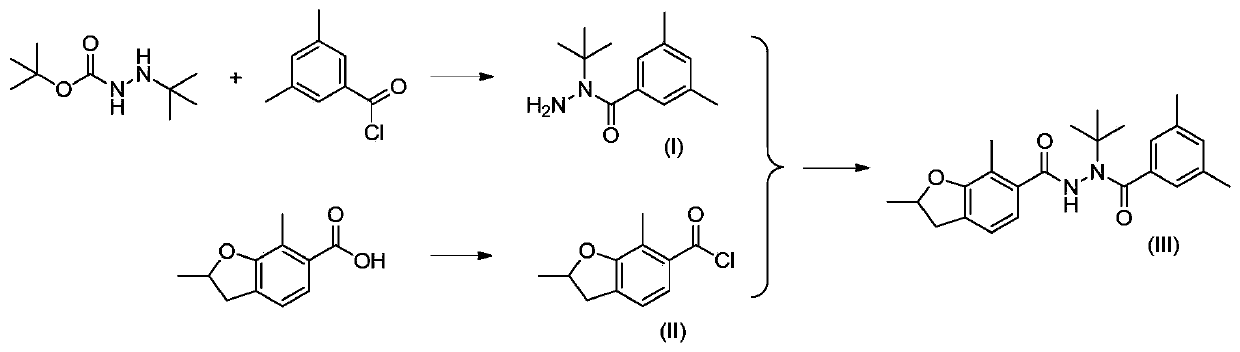
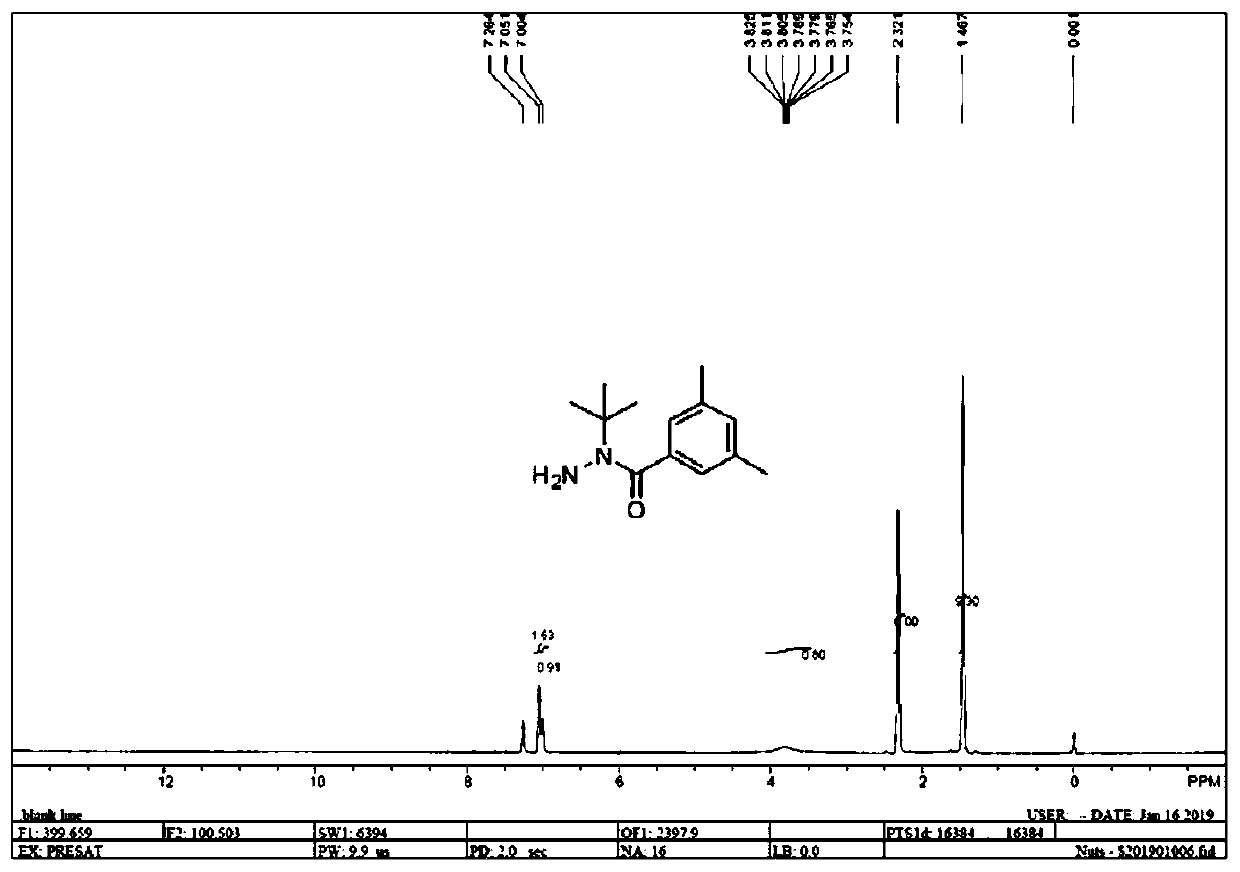
[0061] Embodiment 1: the synthesis of N-(3,5-dimethylbenzoyl)-N-tert-butylhydrazine (I)
[0062] Add tert-butoxycarbonyl-2-tert-butylhydrazine (189g, 1.00mol) and toluene (600ml) into the reaction flask, cool down to 5-8°C, stir, and dropwise add 3,5-dimethylbenzoyl chloride (168g , 0.99mol) and sodium hydroxide aqueous solution (mass concentration is 30%, 132g, 0.99mol), the dropwise process control temperature is lower than 10 ℃. After the addition, the reaction was continued for 2 hours. Cool to 0°C and filter. The filter cake was washed twice with water. Add methanol (900ml) to the filter cake and stir to heat up to 30°C, add dropwise concentrated hydrochloric acid (180g, 1.77mol) with a mass concentration of 36%, control the reaction temperature at 30-35°C, and continue the reaction for 6 hours after the addition. Add sodium carbonate to adjust the pH to 6-8, filter, wash the filter cake twice with water, and dry to obtain N-(3,5-dimethylbenzoyl)-N-tert-butylhydrazine ...
Embodiment 2
[0064] Example 2: Synthesis of N-(3,5-dimethylbenzoyl)-N-tert-butylhydrazine (I)
[0065] Add tert-butyl 2-tert-butylcarbazinate (189g, 1.00mol) and toluene (600ml) into the reaction flask, cool down to 5-8°C, stir, and dropwise add 3,5-dimethylbenzoyl chloride (168g , 0.99mol) and sodium hydroxide aqueous solution (mass concentration is 30%, 132g, 0.99mol), dropwise process control temperature is lower than 10 ℃, after adding, continue insulation reaction for 2 hours, cool to 0 ℃, filter. Wash the filter cake twice with water, add ethanol (900ml) to the filter cake and stir the temperature to 30°C, add dropwise concentrated hydrochloric acid (36%, 180g, 1.77mol) with a mass concentration of 36%, and control the reaction temperature at 30-35°C. After the addition was complete, the reaction was continued for 6 hours. After the reaction, add sodium carbonate to adjust the pH to 6-8, filter, wash the filter cake twice, and dry to obtain N-(3,5-dimethylbenzoyl)-N-tert-butylhydraz...
Embodiment 3
[0066] Example 3: Synthesis of N-(3,5-dimethylbenzoyl)-N-tert-butylhydrazine (I)
[0067] Add tert-butoxycarbonyl-2-tert-butylhydrazine (189g, 1.00mol) and toluene (600ml) into the reaction flask, cool down to 5-8°C, stir, and dropwise add 3,5-dimethylbenzoyl chloride (130g , 0.80mol) and sodium hydroxide aqueous solution (mass concentration is 30%, 107g, 0.80mol), the dropwise addition process controls the temperature -10 ℃. After the addition, the reaction was continued for 2 hours. Warm up to 0°C and filter. The filter cake was washed twice with water. Add methanol (900ml) to the filter cake and stir to heat up to 30°C, add dropwise concentrated hydrochloric acid (180g, 1.77mol) with a mass concentration of 36%, control the reaction temperature at 40-50°C, and continue the reaction for 5 hours after the addition is complete. Add sodium carbonate to adjust the pH to 6-8, filter, wash the filter cake twice with water, and dry to obtain N-(3,5-dimethylbenzoyl)-N-tert-butylh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com