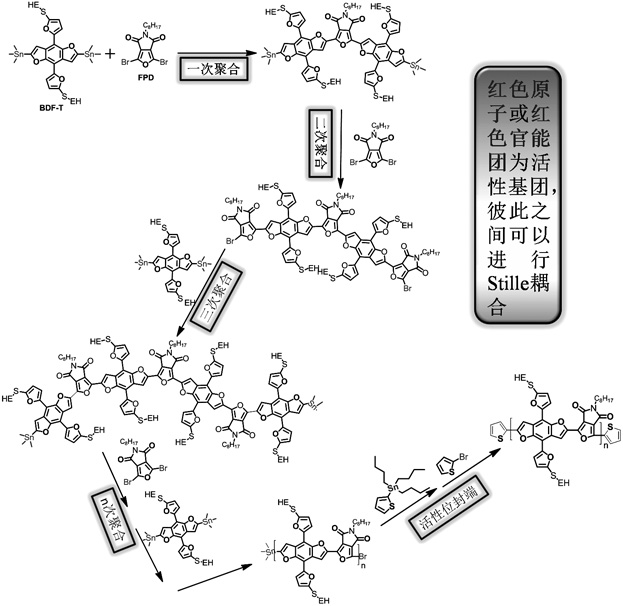
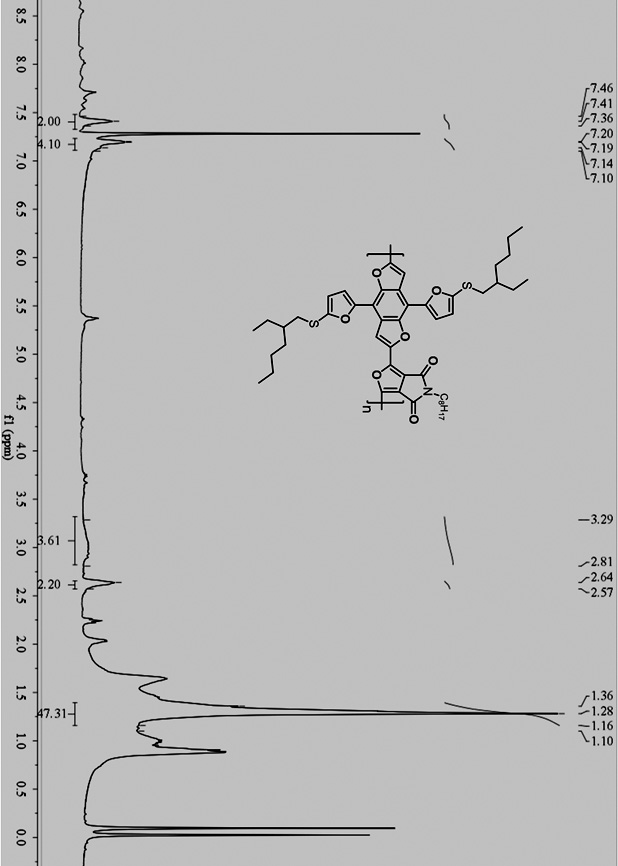
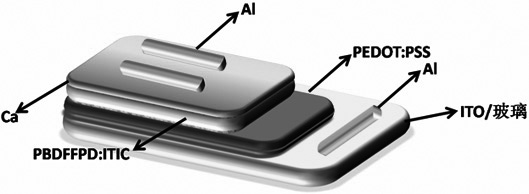
A kind of whole furan skeleton donor-acceptor conjugated polymer, its preparation method and the organic solar cell prepared by using it
A technology of conjugated polymers and solar cells, applied in the fields of material chemistry and electrochemistry, can solve the problems of unfavorable large-scale production and application of organic solar cells, cumbersome synthesis steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
[0049] Synthesis of 2,5-dibromo-3,4-furandicarboxylic acid (1)
[0050] Add furan-3,4-dicarboxylic acid (2.56 mmol, 20.4 g) and 15 mL of glacial acetic acid into a 25.0 mL three-necked flask equipped with a constant pressure dropping funnel, a reflux condenser and a tail gas absorption device, and raise the temperature to 25 °C and stir 0.5 hours, cooled to room temperature. Liquid bromine (36.0 mmol, 1.84 mL) was slowly added dropwise. After the addition was complete, the mixture was stirred at room temperature for 1.0 h, warmed to reflux and stirred overnight. Cool to room temperature, spin out most of the solvent under reduced pressure, add a small amount of deionized water, and slowly add sodium thiosulfate until the reaction no longer produces gas, filter, and rinse the filter cake with a small amount of deionized water three times. The obtained solid was added into deionized water for recrystallization, filtered and dried under vacuum at 65 °C for 48 h to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com