A d-a type conjugated polymer containing fused ring lactone and its preparation method and application
A conjugated polymer, D-A technology, applied in the field of electrochemical materials, can solve problems such as not giving full play to the value of ester groups, affecting photoelectric performance, and increasing steric hindrance, etc., to achieve excellent light absorption and carrier transport performance, widening the conjugate plane, and reducing steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] Synthesis of D-A Conjugated Polymer P1 Containing Fused Lactone
[0141] The synthetic route is:
[0142]
[0143] (1) Synthesis of intermediate B1
[0144] Add 5H-dithieno[3,2-B:2',3'-D]pyran-5-one (150mg), N-bromosuccinimide (NBS) (746mg ), chloroform (10mL) and N,N-dimethylformamide (DMF) (5mL), react at room temperature in the dark for 48h. The chloroform was removed by rotary evaporation under reduced pressure, and the crude product was purified by column chromatography, using a mixed solvent of dichloromethane and sherwood oil with a volume ratio of 1:2 as an eluent to obtain a white solid product B1 (214mg, yield 81% ).
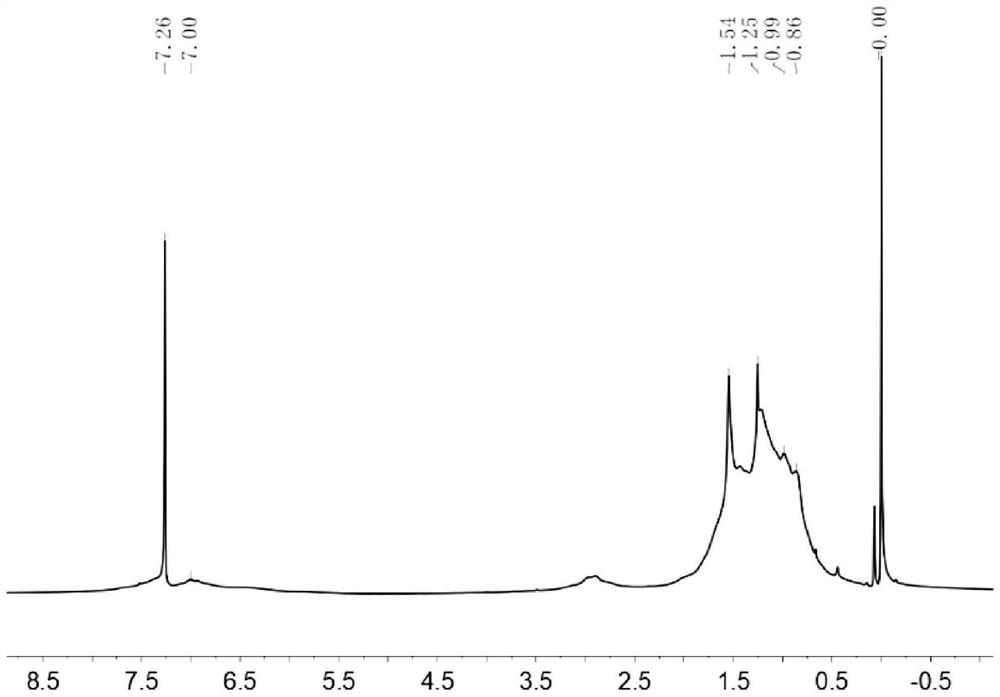
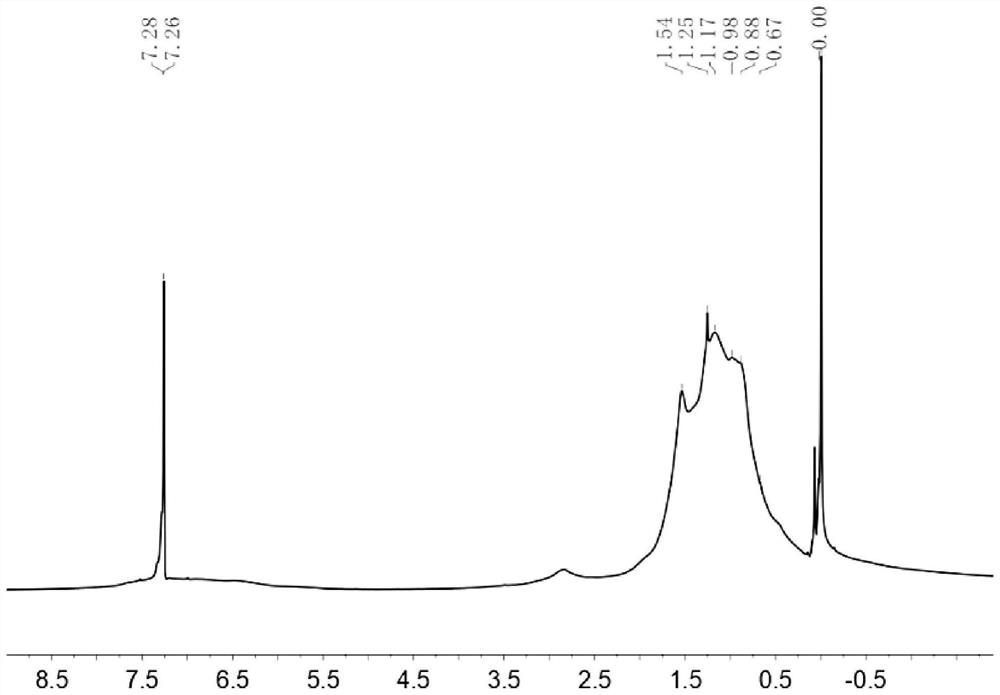
[0145] NMR and MS data of intermediate B1: 1 H NMR (CDCl 3 ,400MHz,δ / ppm):7.57(s,1H),7.11(s,1H). 13 C NMR (CDCl 3 ,100MHz,δ / ppm):155.88,151.39,145.40,128.86,122.33,121.11,115.71,113.67,111.97.Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS)(m / z):366.04(M + ).
[0146] (2) Synthesis of interm...
Embodiment 2
[0157] Synthesis of D-A Conjugated Polymer P2 Containing Fused Lactone
[0158] The synthetic route is:
[0159]
[0160] Steps (1), (2) and (3) of this embodiment are the same as in Embodiment 1.
[0161] (4) Synthesis of D-A type conjugated polymer P1 containing fused ring lactone
[0162] Add D1 (64 mg), (4,8-bis(5-(2-ethylhexyl)-4-fluorothiophen-2-yl)benzo[1,2-b:4,5 -b']dithiophene-2,6-diyl)bis(trimethylstannane) (compound E1) (70mg), Pd 2 (dba) 3 (2.72mg) and P(o-Tol) 3 (7.24mg), reacted at 110°C for 24h under the protection of nitrogen. After cooling to room temperature, the reaction solution was added dropwise to 150 mL of methanol for chromatography, and the precipitate was filtered to obtain the crude product, which was transferred to a Soxhlet extractor, extracted with n-hexane, dichloromethane and chloroform in sequence, and concentrated Finally, the obtained chloroform extract was obtained, which was dropped into methanol for further chromatography, and fi...
Embodiment 3
[0166] Synthesis of D-A Conjugated Polymer P3 Containing Fused Lactone
[0167] The synthetic route is:
[0168]
[0169] (1) Synthesis of Intermediate B3
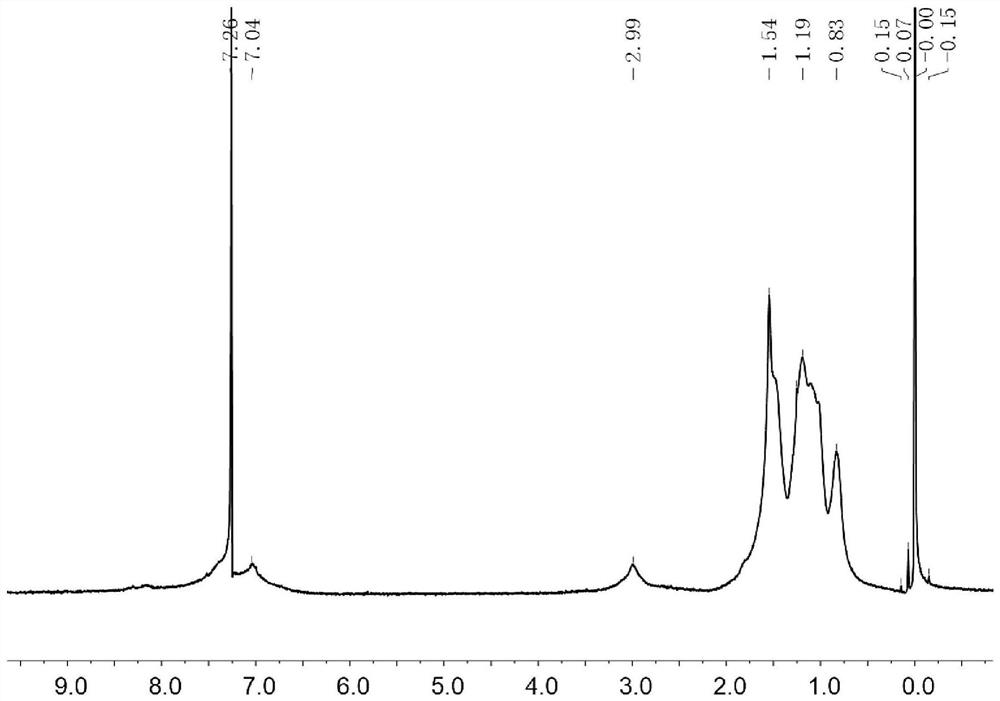
[0170] Add 2,7-dibromo-9-fluorenone (501.4mg), sodium perborate monohydrate (2961.2mg) and trifluoroacetic acid (40mL) sequentially into a 200mL Schlenck tube, and react in the dark at 68°C for 12 hours under nitrogen protection . The trifluoroacetic acid was removed by rotary evaporation under reduced pressure, and the crude product was purified by column chromatography, using a mixed solvent of dichloromethane and sherwood oil with a volume ratio of 2:1 as an eluent to obtain a white solid product B3 (446mg, yield 85%). Intermediate B3 mass spectrometry data: MALDI-TOF MS (m / z): 354.0 (M + ).
[0171] (2) Synthesis of intermediate C3
[0172] Intermediate B3 (113 mg), (4-(2-butyloctyl)thiophene-2-trimethyltin reagent (397.5 mg), Pd(PPh 3 ) 4 (29.5mg) and toluene (10ml), refluxed overnight under nitrogen protecti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com