Spirobifluorene fluorescence probe as well as preparation method and application thereof
A fluorescent probe, spirobifluorene technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve the effect of wide detection range, high sensitivity and selectivity, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
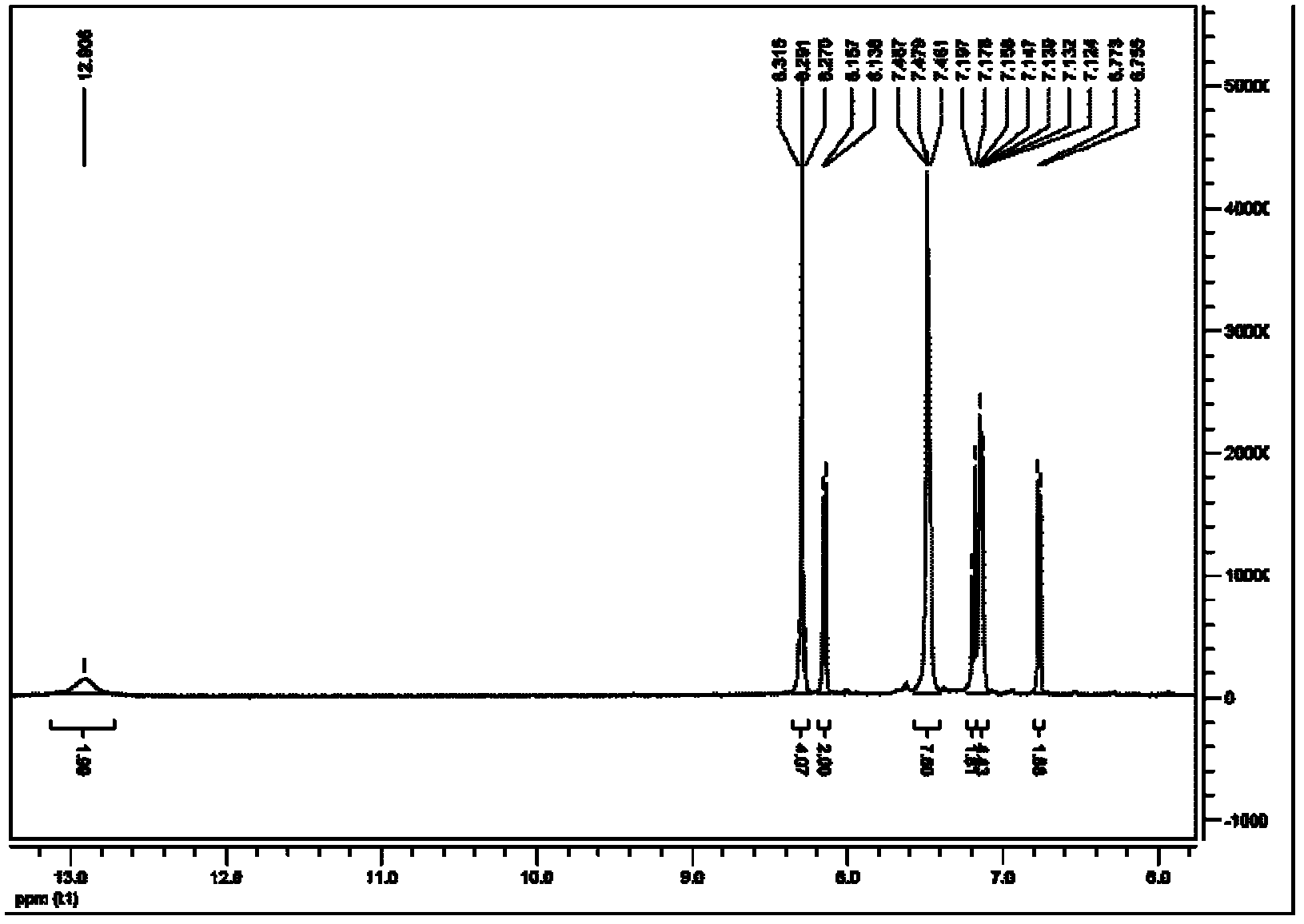
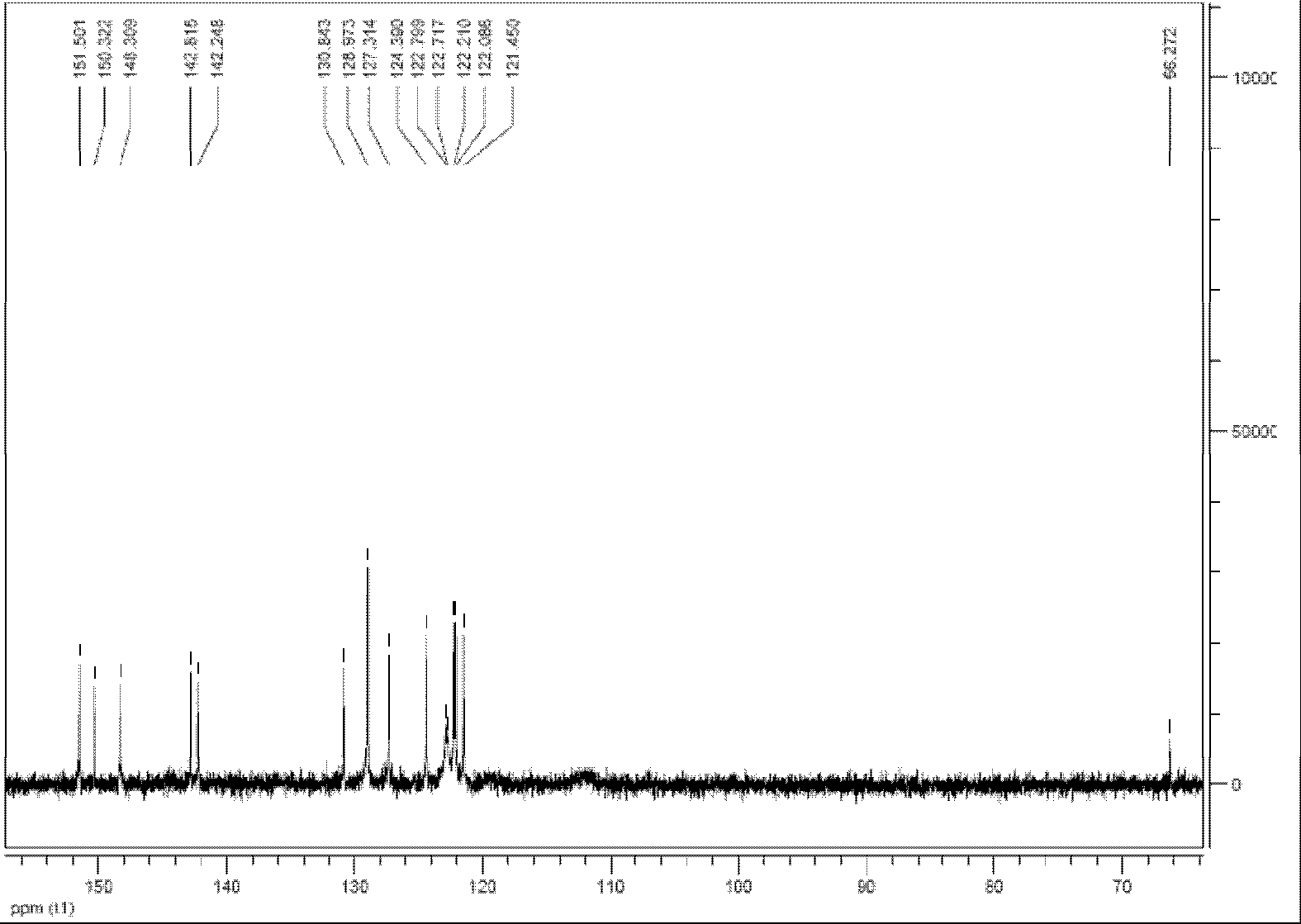
Image
Examples
Embodiment 1
[0062] 1), the synthesis of 4,4'-dimethylbiphenyl
[0063] Under nitrogen protection, successively weigh 3g (125mmol) of dry magnesium powder, 0.2g (1.2mmol) of ferric chloride in a 100ml dry three-necked flask, then weigh 11g (64.3mmol) of p-bromotoluene in 50ml and dry in a constant pressure dropping funnel. Inject 60 ml of anhydrous THF into the three-necked flask, and vigorously stir at room temperature. First add 5% p-bromotoluene rapidly and dropwise after the reaction is initiated. After the dropwise addition was completed, the reaction was continued for 30 min, the reaction was stopped, cooled to room temperature, and filtered with suction to obtain a black filtrate, and the solvent was removed to obtain a black paste. Add 50ml of dichloromethane and 30ml of water in sequence, vibrate vigorously, and a lot of flocs appear in the solution. Slowly add dilute hydrochloric acid solution dropwise to the solution and shake continuously until the flocs disappear.
[0064]...
Embodiment 2
[0074] Embodiment 2 Compound (I) to the selectivity of silver ion (1)
[0075] Using the compound (I) synthesized above, the selectivity to silver ions was evaluated by fluorescence method. Compound (I) is dissolved in acetonitrile: water=15:1 (volume ratio) in the mixed solvent, makes its concentration be 1 * 10 -5 M. Then add different metal ions respectively, so that the concentration of metal ions is 1×10 -4 M, to test its fluorescence emission spectrum. Fluorescence test conditions are: excitation wavelength 360nm, slit width 5 / 5, voltage 420V. The results are shown in image 3 middle. From image 3 It can be seen that compound (I) has high selectivity to silver ions (dotted line is silver ion, and the rest are successively Li + 、Na + , Ca 2+ 、Co 2+ 、Cd 2+ 、Cu 2+ , Pb 2+ , Mg 2+ , Mn 2+ , Hg 2+ and blank control). When the compound (I) is combined with silver ions, the fluorescence intensity of the solution is rapidly weakened to almost zero. However, af...
Embodiment 3
[0076] Embodiment 3 compound (I) to the selectivity (2) of silver ion
[0077] Using the compound (I) synthesized above, the selectivity to silver ions was evaluated by the method of absorption spectrum. Compound (I) is dissolved in acetonitrile: water=15:1 (volume ratio) in the mixed solvent, makes compound (I) concentration be 1 * 10 -5 M, and then add different metal ions, so that the concentration of metal ions is 1×10 -4 M, the absorption spectrum of test compound (I). The results are shown in Figure 4 Middle (dashed line is silver ion, the rest are Li + 、Na + , Ca 2+ 、Co 2+ 、Cd 2+ 、Cu 2+ , Pb 2+ , Mg 2+ , Mn 2+ , Hg 2+ and blank control). From Figure 4 It can be seen that compound (I) has high selectivity to silver ions, and when compound (I) is combined with silver ions, the absorbance of the solution is rapidly weakened. However, after compound (I) is mixed with other metal ions, the absorbance hardly changes. The abscissa is the wavelength (nm), and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com