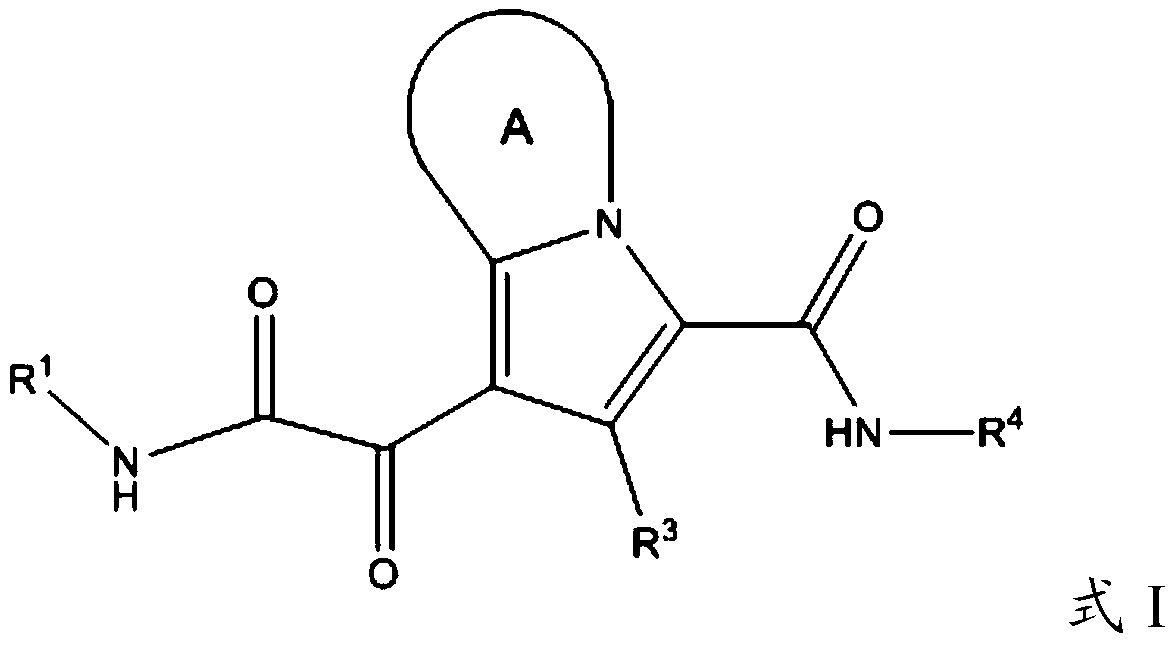
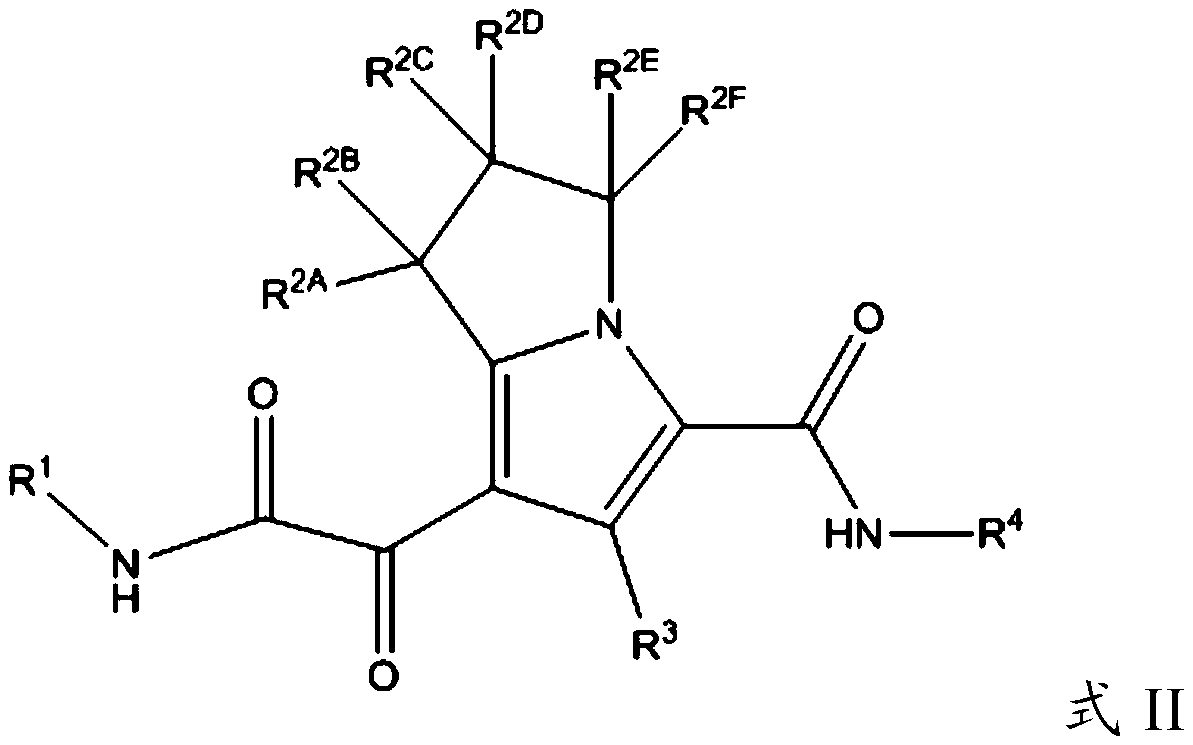
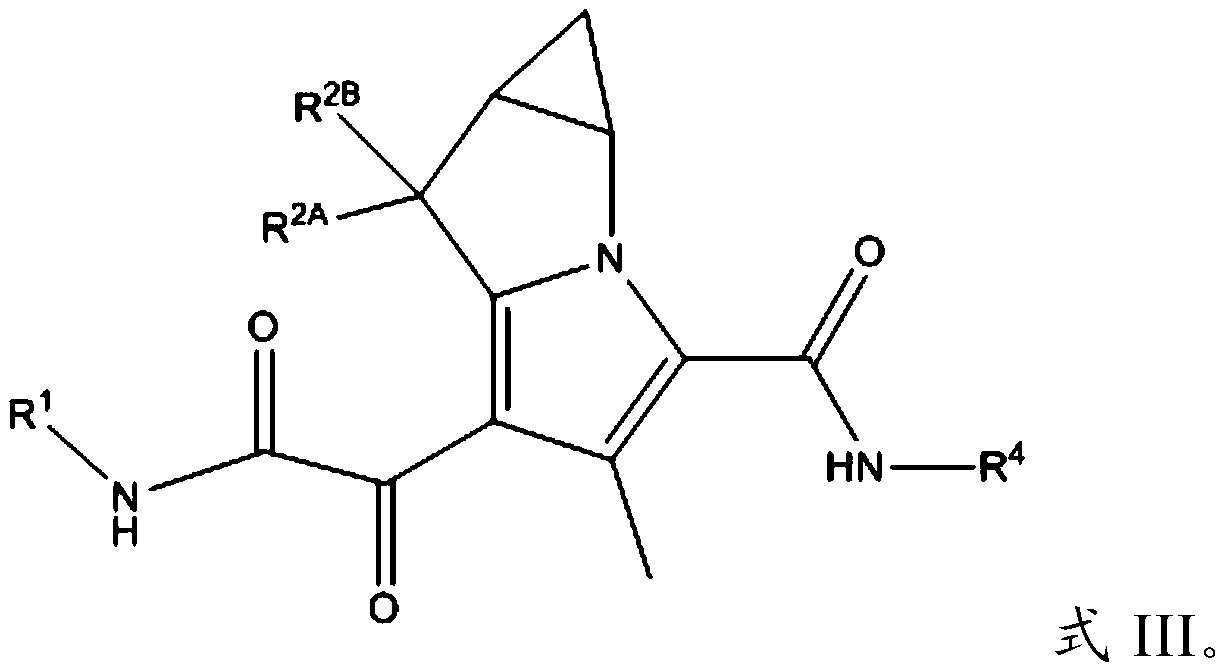
Substituted pyrrolizine compounds and uses thereof
A compound, haloalkyl technology, applied in the field of pyrrolizine compounds, can solve the problem of not clearing HBV infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0415] Example 1 : 7-(2-(tert-butylamino)-2-oxoacetyl)-6-chloro-N-(3-cyano-4-fluorophenyl)-2,3-dihydro-1H-pyrrole Oxazine-5-carboxamide (1)
[0416]
[0417] step 1 To a reaction tube with a stirrer was added methyl 3-chloro-1H-pyrrole-2-carboxylate (0.32 g, 2.0 mmol), norbornene (0.38 g, 4.0 mmol), potassium bicarbonate (0.60 g, 6.0 mmol), bis(acetonitrile)palladium(II) dichloride (0.052g, 0.2mmol) and (3-bromopropoxy)(tert-butyl)diphenylsilane (1.51g, 4mmol) in anhydrous dimethyl Acetamide (2 mL) was heated at 90°C for 24 hours. Ethyl acetate (100 mL) was added and filtered. The filtrate was washed with water, brine and dried over sodium sulfate. Removal of solvent and purification of the residue by column (0-80% ethyl acetate in hexanes) gave 1-(3-((tert-butyldiphenylsilyl)oxy)propyl)-3-chloro - 1H-pyrrole-2-carboxylic acid methyl ester.
[0418] Step 2: To 1-(3-((tert-butyldiphenylsilyl)oxy)propyl)-3-chloro-1H-pyrrole-2-carboxylic acid methyl ester (0.72g, 1....
Embodiment 2
[0425] Example 2 : (R)-N-(3-chloro-4-fluorophenyl)-6-methyl-7-(2-oxo-2-((1,1,1-trifluoroprop-2-yl) Amino)acetyl)-2,3-dihydro-1H-pyrrolazine-5-carboxamide (2)
[0426]
[0427] step 1 : In dichloromethane (150mL) solution of L-proline benzyl ester hydrochloride (8.83g, 36.5mmol) and N-ethyldiisopropylamine (13mL, 75mmol) cooled to 0°C, oxychloride was added dropwise Methyl acetate (5.0 mL, 54 mmol). The reaction mixture was warmed to ambient temperature and stirred for 1 h at which time the reaction mixture was quenched by pouring into cooled saturated aqueous sodium bicarbonate. The aqueous phase was extracted three times into dichloromethane, the combined organic phases were washed with brine, dried over sodium sulfate, filtered, and the solvent was removed under reduced pressure to give (2-methoxy-2-oxoacetyl)-L - Proline benzyl ester, which was carried forward without further purification.
[0428] step 2 : (2-methoxy-2-oxoacetyl)-L-proline benzyl ester (10.6g, ...
Embodiment 3
[0436] Example 3 .N-(3-chloro-4-fluorophenyl)-7-(2-((3,3-difluoro-1-(methylcarbamoyl)cyclobutyl)amino)-2-oxoacetyl Base) -6-methyl-2,3-dihydro-1H-pyrrolizine-5-carboxamide (3)
[0437]
[0438] Using 1-amino-3,3-difluoro-N-methylcyclobutane-1-carboxamide hydrochloride instead of R-trifluoroisopropylamine, synthesize N-(3-chloro -4-fluorophenyl)-7-(2-((3,3-difluoro-1-(methylcarbamoyl)cyclobutyl)amino)-2-oxoacetyl)-6-methyl -2,3-Dihydro-1H-pyrrolazine-5-carboxamide (3).
[0439] Synthesis of 1-amino-3,3-difluoro-N-methylcyclobutane-1-carboxamide hydrochloride
[0440]
[0441] step 1 .To 1-amino-3,3-difluorocyclobutane-1-carboxylic acid (990mg, 6.55mmol) in methanol (8mL) solution at 0°C was added 1M aqueous sodium hydroxide solution (7mL, 7mmol), followed by Di-tert-butyl dicarbonate (1.8 g, 8.2 g). The reaction mixture was warmed to ambient temperature, stirred for 14 hours, acidified with dilute aqueous hydrogen chloride, and extracted into ether. The ether phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com