Test strip for testing residual diazepam
A technology of test strips and stability, which is applied in the field of colloidal gold test strips for the detection of stable drug residues. It can solve the problems of complex processing, environmental pollution, and long detection time, and achieve the effects of simple operation, high sensitivity, and short detection time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Preparation of Stability Detection Test Paper Card
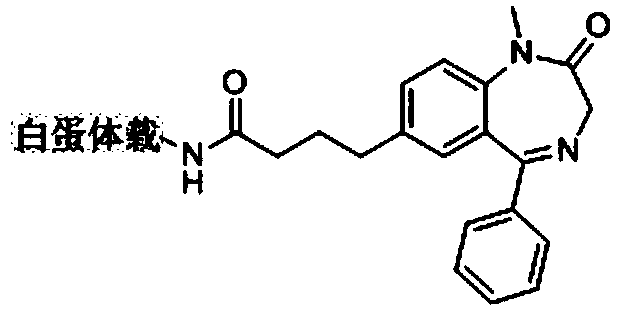
[0046] 1. Synthesis and identification of diazepam hapten and diazepam hapten-carrier protein conjugate
[0047] (1) Synthesis of stable hapten
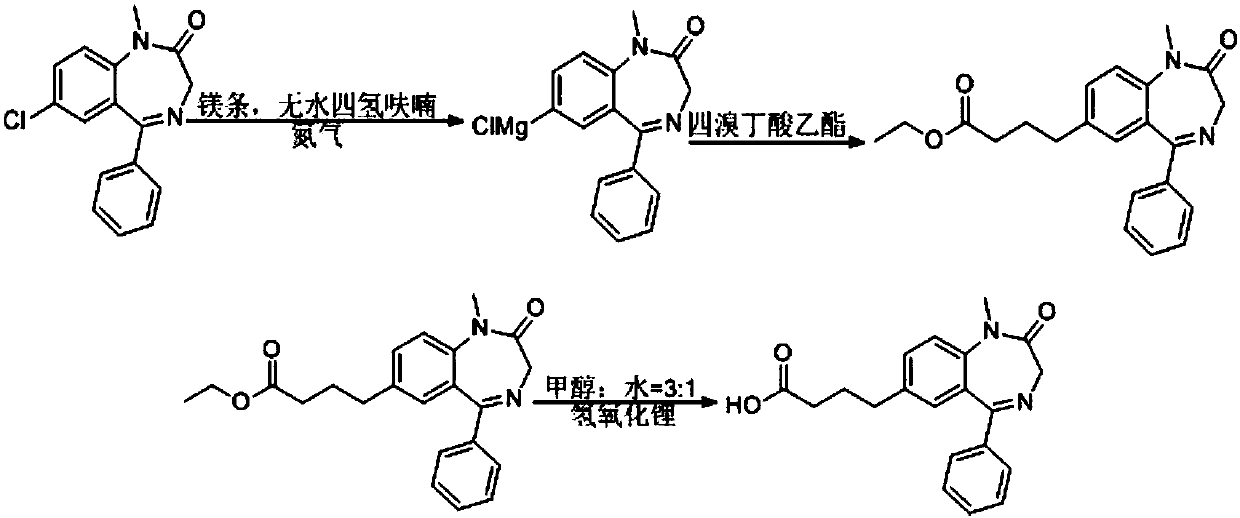
[0048] Weigh 5g of diazepam raw material, dissolve 5g of diazepam and an appropriate amount of magnesium in an appropriate amount of tetrahydrofuran, react under nitrogen protection for 2-3h, add ethyl tetrabromobutyrate dropwise, and react for another 2-3h;
[0049] TLC thin-layer plate detection reaction, until there is no raw material or the raw material point is very shallow, the reaction is terminated;
[0050]Silica gel column purification, concentration to obtain the product, which is 4-bromobutyric acid ethyl ester derivative, hydrolyzed to obtain stable hapten;
[0051] The reaction technical route is as follows:
[0052]
[0053]
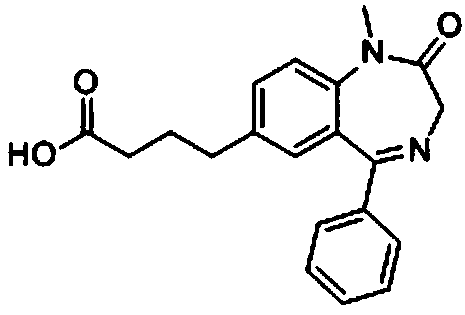
[0054] The molecular structural formula of the hapten obtained by the technical route is shown in Formula I:
[005...
Embodiment 2
[0085] Example 2 Detection of Stability Residues in Samples
[0086] 1. Sample pretreatment
[0087] (1) Animal tissue pretreatment
[0088] Weigh 2.0±0.05g of homogenized tissue samples into a centrifuge tube, add 2mL of deionized water containing 10% methanol, and cap the bottle tightly. Place the centrifuge tube containing the sample in a water bath at 80°C for 10 minutes, absorb more than three drops of the solution and pour it into a 1.5mL centrifuge tube. If there is obvious yellow turbidity, please centrifuge, and then use the supernatant as the sample solution to be tested.
[0089] (2) Pretreatment of urine samples
[0090] Put a small amount of urine sample in a centrifuge tube, add 1mL of deionized water, and mix it as the sample solution to be tested.
[0091] 2, detect with the test paper card of the present invention
[0092] Use a dropper to drop 3 drops of sample into the hole of the reagent card, and observe the result after 5-8 minutes. If it exceeds 10...
Embodiment 3
[0095] Example 3 Example of sample detection
[0096] Take 20 samples each of pork, chicken, and urine and 20 samples of various negative samples with known stable drug residual concentration greater than 5ng / g, and calculate the negative and positive rates. The results are shown in Table 1-Table 3.
[0097] Table 1 Negative and positive rate detection of pork samples
[0098] batch
[0099] Table 2 Detection of negative and positive rate of chicken samples
[0100] batch
[0101] Table 3 Negative and positive rate detection of urine samples
[0102] batch
[0103] The results show that there are 20 positive samples and 20 negative samples in pork, chicken, and urine samples. The negative and positive coincidence rates in pork samples are 100%, and the false positive rate in chicken samples is less than 7%, and the positive coincidence rate is 100%. %, the negative coincidence rate was above 90%. The false positive rate in the urine sample is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com