Tri pyrazole ligand based nickel metal organic framework material, and preparation method and applications thereof
A technology of organic framework and three-headed pyrazole, which is applied in the field of crystalline materials, can solve problems such as difficulty in obtaining crystals, difficulty in reversible self-repair of coordination bonds, difficulty in forming MOFs single crystals, etc., and achieves the effect of good alkali stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] First, 4-bromoacetophenone (20.0mmol) was dissolved in ethanol solution (30mL), and thionyl chloride (40.0mmol) was slowly added dropwise to obtain 4,4"-dibromo-5' -(4-bromophenyl)-1,1':3',1"-terphenyl;
[0029] Under nitrogen protection, 4,4"-dibromo-5'-(4-bromophenyl)-1,1':3',1"-terphenyl (20.0mmol), 1-THP-4-pyrazole Boric acid pinacol ester (75.0mmol) and 1,4-dioxane (300mL) and water (100mL) were added in the 500mL round bottom flask, added magnetic sub-stirring, then added K 2 CO 3 (90.0mmol) and Pd(PPh 3 ) 4 (3.0 mmol), and the reaction system was stirred at 100° C. for 24 hours. After the reaction was completed, the solvent in the reaction system was spin-dried, and the residue was dissolved in ethyl acetate (300mL), washed with water (300mL×2) and saturated brine (300mL) successively, washed over anhydrous Na 2 SO 4 After drying, filter and concentrate. The crude product was purified by column chromatography (SiO 2, petroleum ether / ethyl acetate=20:1~1:1...
Embodiment 2
[0031] The white solid obtained in the previous step was dissolved in hydrochloric acid ethanol solution (2mol L -1 , 300 mL), stirred at 50°C for 24 hours, and after the reaction was completed, the organic solvent was removed by rotary evaporation. The remaining solid was dispersed in 300 mL of water, and then the pH was adjusted to 10 by adding a saturated sodium carbonate aqueous solution dropwise. The resulting suspension was filtered, the solid was washed with water (300mL×3), and dried in vacuum at 60°C to obtain a white solid 4,4'-(5'-(4-(1H-pyrazol-4-yl)benzene base)-[1,1':3',1"-tetraphenyl]-4,4"-diyl)bis(1H-pyrazole)(H 3 BTPP, 65% yield).
Embodiment 3
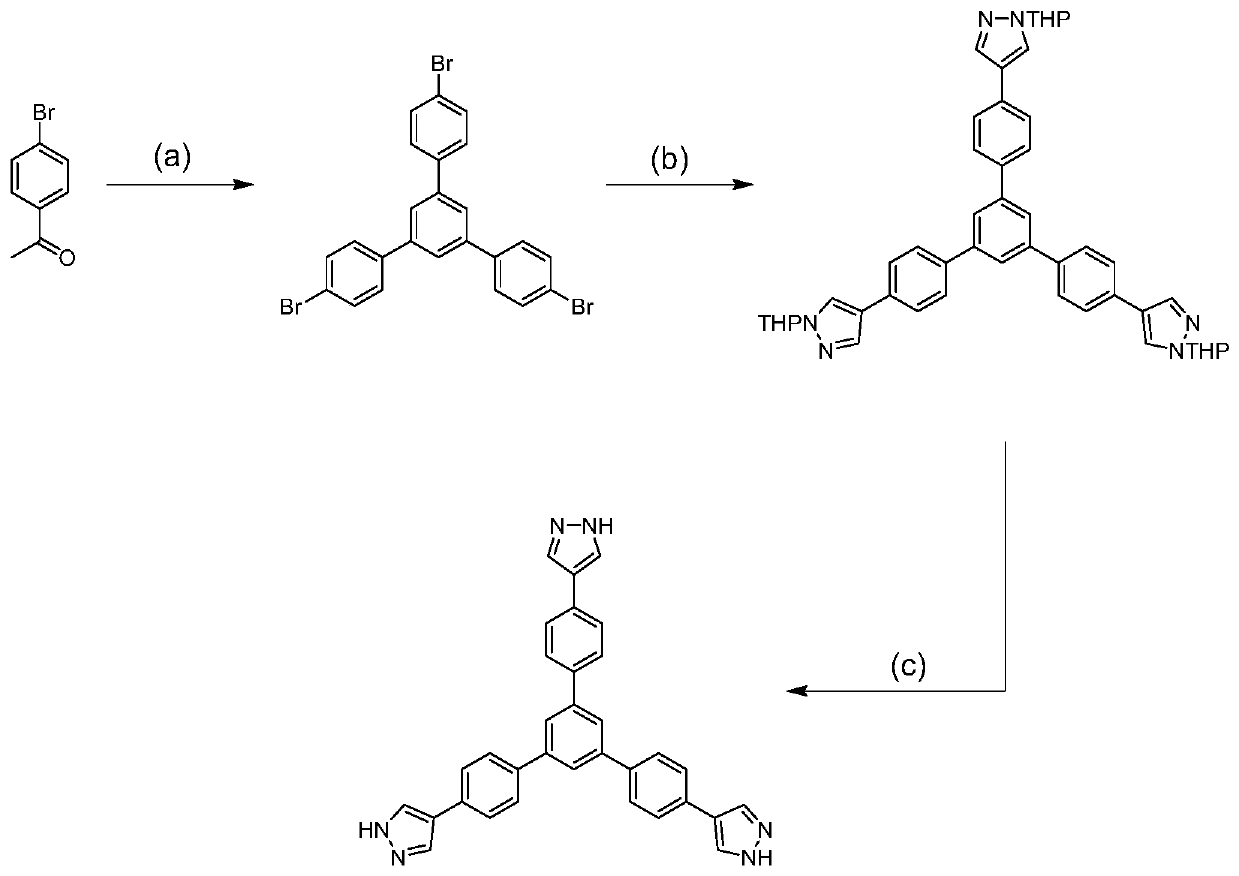
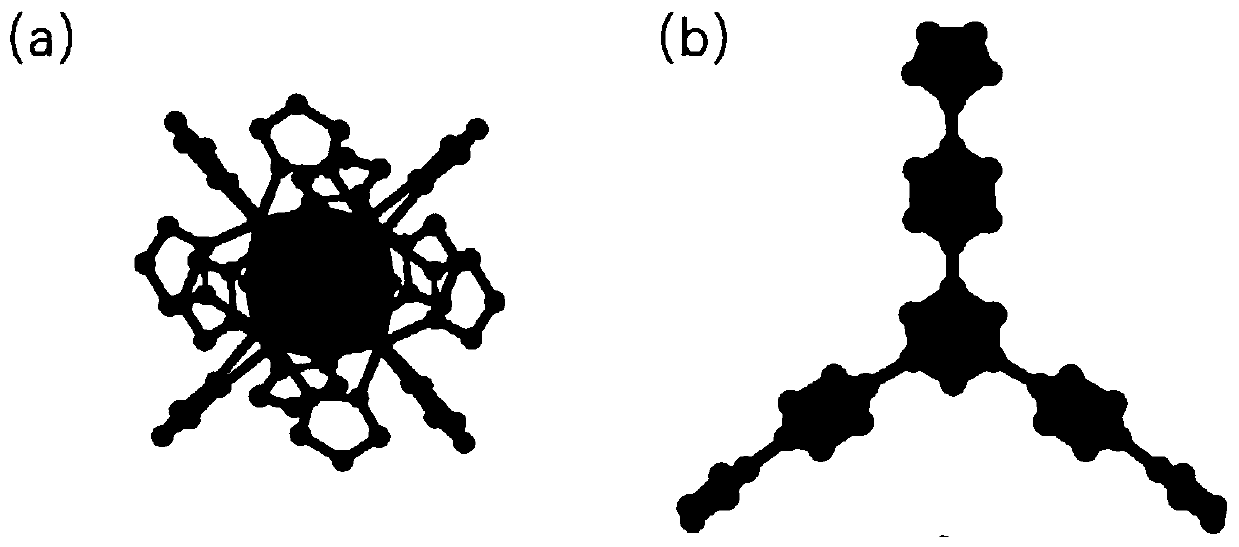
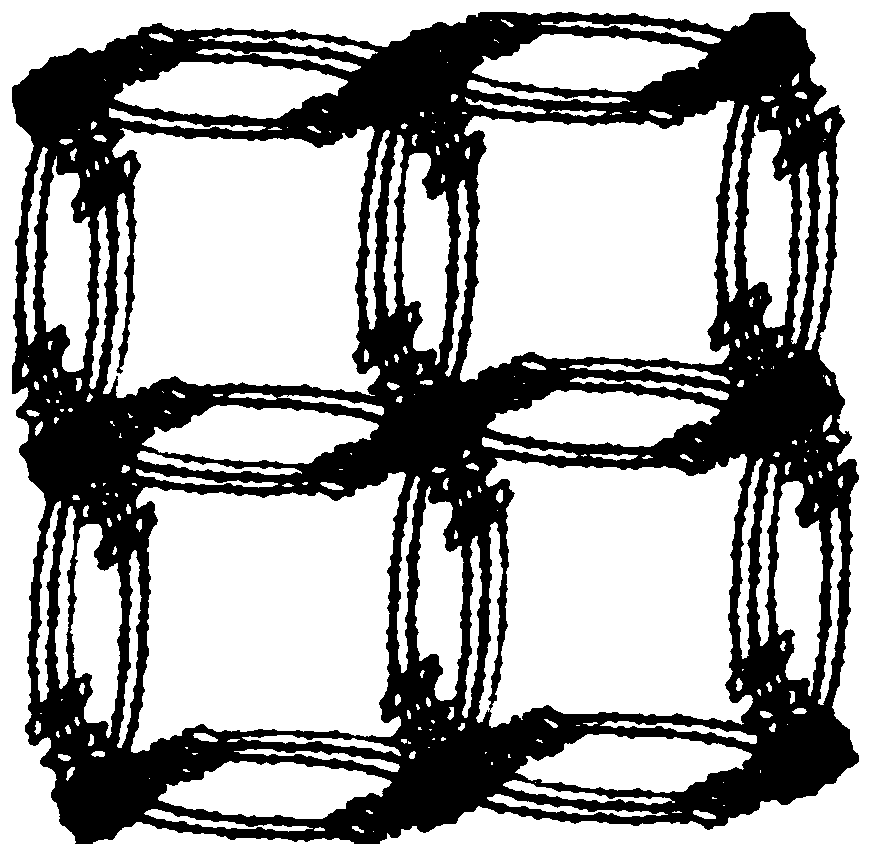
[0033] Weighing 1,3,5-H 3 BTPP(0.025mmol) and Ni(NO 3 ) 2 ·6H 2 O (0.05mmol, 14.5mg) was put into a 25mL beaker, 3mL of DMA solution and 3mL of deionized water were added, and then the beaker was sealed and put into an ultrasonic instrument, and ultrasonicated at room temperature for 5 minutes. After the end, the solution was transferred to 20mL polytetrafluoroethylene reactor. After sealing, the reaction kettle was placed in an oven at 120° C. for 48 hours to react. Close the oven after the reaction is over, open the reaction kettle after cooling to room temperature, filter and collect the solid particles obtained in the reaction kettle, and then use DMA, H 2 Washed with O and EtOH (5mL×3), observed under a microscope to obtain blue prism crystals (Ni 8 (OH) 4 (OH 2 ) 2 (BTPP) 4 ), (yield: 72%, based on 1,3,5-H 3 BTPP ligand).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com