Benzoxazine resin containing quaternary ammonium group and its preparation method and application
A technology of quaternary ammonium group and benzoxazine, which is applied in the field of main chain type benzoxazine resin and its preparation, can solve the problems of reduced electrical conductivity and high price, and achieve excellent alcohol resistance, low cost, and excellent alkali stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1 Synthesis of quaternary ammonium group-containing main chain type benzoxazine based on bisphenol A and 4,4'-diaminodiphenylmethane
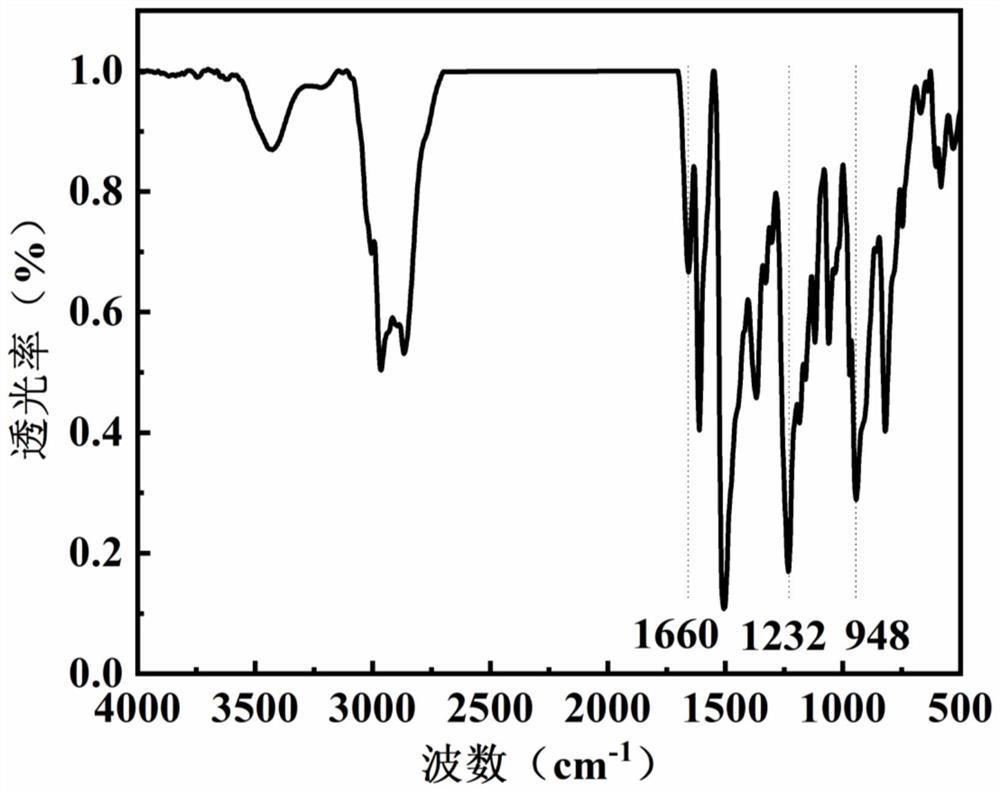
[0105] Dissolve 1.1g of bisphenol A, 2.0g of 4,4'-diaminodiphenylmethane, 1.6g of 4-(2-dimethylaminoethyl)phenol, 1.4g of formaldehyde and 0.2g of triethylamine in 30ml of dimethyl In sulfoxide, the temperature was raised to 90°C for 5h. Post-processing: remove the solvent under reduced pressure, wash with methanol, ethanol, and n-hexane in turn, and dry in vacuum at 70°C overnight to obtain the main chain type benzoxazine.
[0106] 1.1 g of main chain type benzoxazine and 0.3 g of methyl iodide were dissolved in 20 mL of dioxane, heated to 80° C. under magnetic stirring and reacted for 3 hours. After the reaction is finished, filter and vacuum-dry the filter cake to obtain a main-chain benzoxazine containing quaternary ammonium groups with a yield of 95%.
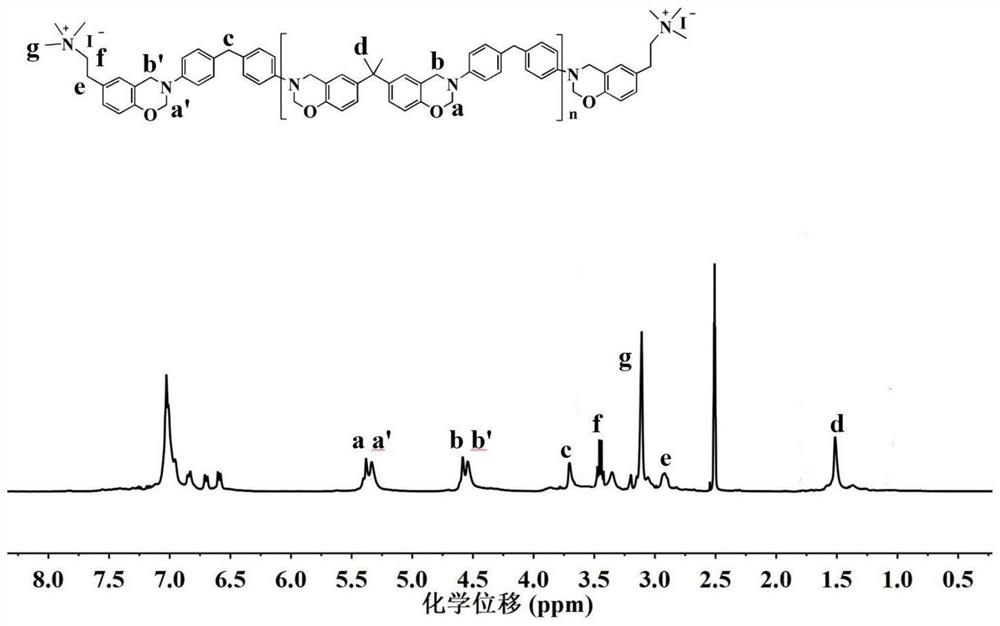
[0107] 1 H NMR (400MHz, d 6 -DMSO, ppm): δ=5.34 (d, J=17.6Hz, O-CH ...
Embodiment 2
[0109] Example 2 Synthesis of quaternary ammonium group-containing main chain type benzoxazine based on bisphenol S and 4,4'-diaminodiphenyl ether
[0110] Dissolve 1.5g of bisphenol S, 2.4g of 4,4'-diaminodiphenyl ether, 1.9g of 4-dimethylaminomethylphenol, 1.8g of formaldehyde and 0.3g of pyridine in 30mL of ethanol, heat up to 85°C for 12 hours . Post-processing: remove the solvent under reduced pressure, wash with methanol, ethanol, and n-hexane in turn, and dry in vacuum at 50°C overnight to obtain the main chain type benzoxazine.
[0111] Dissolve 1.5 g of main-chain benzoxazine and 0.6 g of bromoethane in 15 mL of dimethyl sulfoxide, raise the temperature to 90° C. under magnetic stirring, and react for 5 hours. After the reaction, filter and vacuum-dry the filter cake to obtain the main chain type benzoxazine containing quaternary ammonium groups, with a yield of 90%.
Embodiment 3
[0112] Example 3 Synthesis of quaternary ammonium group-containing main chain type benzoxazine based on bisphenol AF and 4,4'-diaminodiphenylsulfone
[0113] Dissolve 1.8g of bisphenol AF, 2.0g of 4,4'-diaminodiphenylsulfone, 1.9g of 4-dimethylaminomethylphenol, 1.8g of formaldehyde and 0.5g of triethylamine in 30mL of toluene, heat up to 90°C for reaction 20h. Post-processing: remove the solvent under reduced pressure, wash with methanol, ethanol, and n-hexane in turn, and dry in vacuum at 60° C. overnight to obtain the main chain type benzoxazine.
[0114] Dissolve 1.9 g of main-chain benzoxazine and 0.9 g of diethyl sulfate in 25 mL of N,N-dimethylformamide, raise the temperature to 90° C. under magnetic stirring, and react for 5 hours. After the reaction, filter and vacuum-dry the filter cake to obtain main-chain benzoxazine containing quaternary ammonium groups, with a yield of 84%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com